Table of Contents
Page created on March 7, 2018. Last updated on December 18, 2024 at 16:56
Summary
- Iron homeostasis is regulated at the level of absorption by hepcidin and IREs and IRPs
- Iron is absorbed as Fe2+ through DMT1 and ferroportin 1
- Iron is stored in ferritin molecules in the body. It’s transported in the blood by transferrin.
Absorption
Iron is found in the body in two forms. Metabolically active iron is currently in use in molecules such as haemoglobin, cytochromes, metalloenzymes, myoglobin, or bound to a protein called transferrin in the blood. Iron is also stored in the body as a component of a protein called ferritin, found in blood, tissue fluids and cells, or a protein called hemosiderin, which is found in macrophages.
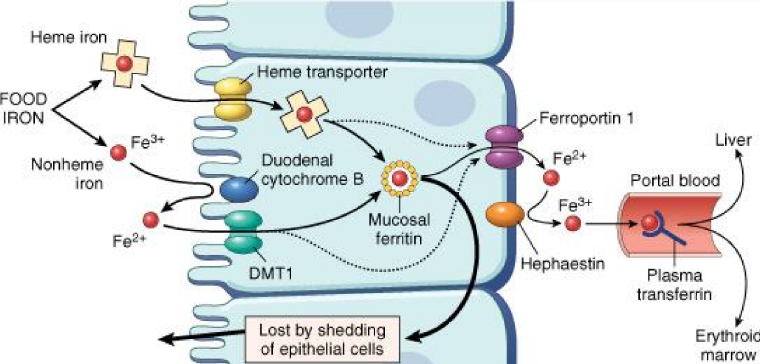
The absorption of iron takes place in the duodenum but begins in the stomach. Gastric acid keeps the iron soluble and in the Fe2+ form, as it’s easier absorbed than the Fe3+ form. Dietary iron can be either inorganic, as a free iron, or as a part of a heme molecule. The two types are absorbed in different ways.
If a Fe3+ ion reaches the duodenum, it’s reduced to Fe2+ by duodenal cytochrome B, a protein found on the surface of the enterocyte. Fe2+ is then absorbed through a transporter called DMT1, or divalent metal transporter 1, which also absorbs lead, zinc and copper ions. Heme molecules are absorbed through a simple heme transporter.
Free iron ions are toxic to the cells, as they create reactive oxygen species through the Fenton reaction. They are therefore stored in a protein called ferritin. Two proteins, called ferroportin 1 and hephaestin, work together to transport the iron from the enterocyte to the blood. Hephaestin is a type of enzyme called ferroxidase, which oxidizes Fe2+ to Fe3+. The ferroportin 1 is the protein that transports the ion out of the cell, while hephaestin oxidizes Fe2+ back to Fe3+ (undoing the work of duodenal cytochrome B). In the blood, Fe3+ binds to a protein called apotransferrin (the apo-prefix means “a protein composed of just polypeptide”, so apotransferrin is the name of the protein itself, before it has bound to iron). The transferrin molecule (apotransferrin + Fe3+) can now travel to wherever it is needed.
Transferrin (Tf) binds to transferrin receptor (TfR) on the target cell. Most cells in the body have this receptor on the cell surface, but it’s most dense on erythrocyte precursors, hepatocytes and placental cells. When transferrin binds to the transferrin receptor, the Tf-TfR complex is transported into the cell, creating a small endosome containing both the receptor and the transferrin. The pH in this endosome is reduced with the help of proton pumps, which allows the iron ions to dissociate from transferrin and escape into the cell.
Iron in the body is stored in ferritin, as mentioned before. One molecule of ferritin can store up to 4500 iron atoms. Note that the protein is only called ferritin when it contains iron. Until it receives iron to store, it’s called apoferritin. Ferritin is mostly found in the liver.
Regulation of iron absorption
The main regulator of iron absorption is a protein called hepcidin. This protein forms complexes with ferroportin, which is then transported into the cell and degraded. A high amount of hepcidin will therefore decrease the amount of ferroportin on enterocytes, which will decrease the amount of iron absorbed.
Several factors influence hepcidin production. Increased iron levels increase its production, as a negative feedback loop. Its production is downregulated in hypoxia, anaemia and insufficient erythropoiesis, all of which make sense logically. It’s also downregulated during inflammation and infection. IL-6 is a signalling molecule during inflammation that is a prominent inducer of hepcidin.
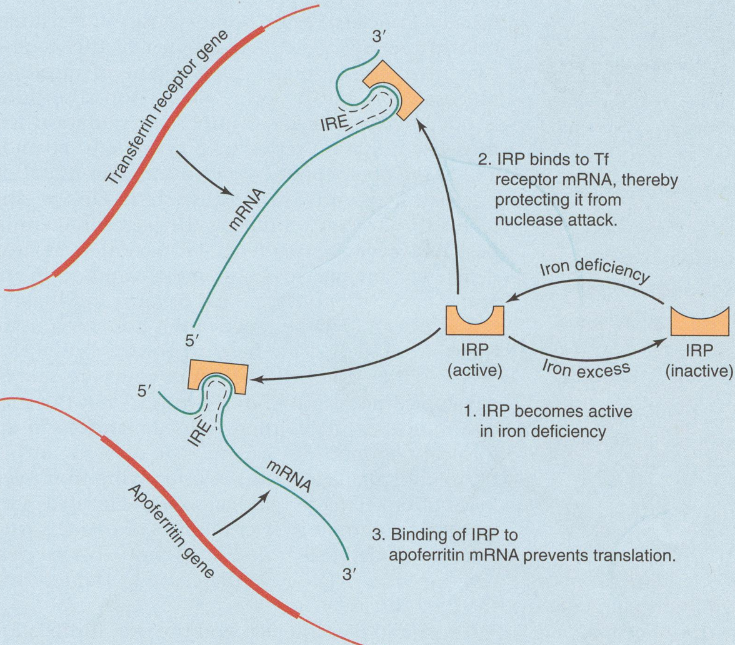
Iron metabolism is also regulated at the expressional level by iron response elements (IREs) and iron response element binding proteins (IRPs), which can bind to the IREs. The latter is activated when iron in the body is low and inactivated when it is high. The mRNA of both apoferritin and transferrin receptor contains IREs. When iron levels are low and IRPs are activated, they bind to IREs on both apoferritin mRNA and transferring mRNA. This binding blocks translation on the apoferritin mRNA, while enhancing translation of the TfR mRNA. This means that when iron levels are low, the amount of ferritin in the cell will be reduced, which will cause less iron to be “stored” and more to be “used”, while the amount of transferrin receptor will increase, which will make the cell take in more transferrin from the blood.
Iron deficiency
Iron deficiency can have many symptoms. It can be caused by blood loss, malabsorption or poor diet. It may lead to anaemia.