Table of Contents
Page created on June 1, 2021. Last updated on June 18, 2024 at 08:31
Huge thanks to Anne Schmidt for writing notes in this subject! I used them a lot when writing these and preparing for the exam, and a lot of these notes are copy-pasted or highly influenced by her notes.
Exam questions in intensive care
1. Definition and emergency treatment of shock
Definition
Shock is a hemodynamic disturbance caused by different diseases, which leads to inadequate oxygen supply in the organs. The ensuing tissue hypoxia causes metabolic disorder in the tissues leading to temporary or permanent functional disorder, and in severe cases to cell death.
Etiology and types
Cardiogenic Shock – cardiac dysfunction leads to inadequate tissue perfusion despite adequate intravascular volume.
- Intrinsic (heart muscle insufficiency)
- MI, low contractility
- Severe tachy-or bradycardia, arrhythmias
- Valve dysfunctions
- Extrinsic
- Tear in septum/ventricle wall
- Extracardiac obstruction
Distributive Shock – vasodilatation of peripheral blood vessels, i.e. blood volume is normal but vascular volume is enlarged. CO increased but SVR decreased.
- Septic shock
- Neurogenic shock
- Anaphylactic shock
- High output cardiac failure
Hypovolaemic shock
- Non-haemorrhagic
- Vomiting/diarrhoea
- Pancreatitis
- Burns
- Adrenal insufficiency
- Haemorrhagic
- GI bleed
- Trauma
- AAA rupture
- Ectopic pregnancy, postpartum bleeding
Obstructive shock – due to extracardiac obstruction
- Cardiac tamponade
- Massive pulmonary embolism
- Tension PTX
Clinical features
- 5 P’s
- Perfusion of skin – pale, cool skin
- Pulse – weak peripheral pulse
- Periphery – capillary refill time > 2 – 3 s
- Pressure – systolic BP < 90 mmHg
- Pee – oliguria (< 1 mL/kg/hour) or anuria (< 0,5 mL/kg/hour)
- Tachycardia
- Tachypnoea, dyspnoea
- Altered mental status
- Ileus
Diagnosis
Clinical diagnosis. Lactate levels can be increased, metabolic acidosis. Can see features of end-organ dysfunction or the underlying cause.
Emergency treatment
General treatment:
- ABC
- Obtain large bore IV access
- Give fluid challenge (give small amount of fluid and check if parameters improve)
- If yes -> Fluid resuscitation with balanced crystalloid – 20 mL/kg
- If no -> vasopressor (norepinephrine) or inotrope (dobutamine)
- Look for and treat underlying cause
Hypovolaemic shock
- Give balanced crystalloid, up to 3 L
- If haemorrhagic -> give blood and colloid in 1:1
- Treat underlying cause
Neurogenic shock
- Cervical spine precaution
- Fluid resuscitation
Anaphylactic shock
- Intubation if airway is compromised (angioedema)
- 0,5 mg epinephrine every 5 minutes
- (Glucocorticoids, antihistamines)
Cardiogenic shock
- Careful with fluids – may cause cardiogenic pulmonary oedema
- Inotropes
- Intra-aortic balloon pump
- Ventricular assist device
- ECMO
- If AMI -> PCI
Obstructive shock
- If tension PTX -> chest puncture at 3th ICS mid-clavicular line or 5th ICS at mid-axillary line
- If PE -> Alteplase or interventional catheterization
2. Syndromes with acute chest pain (aortic dissection, acute myocardial infarction, pneumothorax)
Aortic dissection
Aortic dissection occurs when a tear in the inner wall of the aorta causes blood to flow between the layers of the wall of the aorta, forcing the layers apart. Risk factors:
- Hypertension
- Atherosclerosis
- Connective tissue disorders (Marfan, Ehlers-Danlos)
- Aortitis (Takayasu arteritis)
Types:
- Stanford type A: Dissection involves the ascending aorta.
- Worse
- Stanford type B: Dissection does not involve the ascending aorta.
Patients present with sudden onset severe pain in chest, back, or abdomen, usually tearing pain. Hypertension or hypotension. Unequal pulse and BP between the upper limbs are characteristic.
X-ray shows widened mediastinum. CT angiography or TEE for stable patients.
Treatment:
- If hypotensive -> give fluids ± vasopressor. Target lower-normal range of BP
- If hypertensive -> give beta blockers ± vasodilator. Target lower-normal range of BP
- If Stanford type A or complicated type B: Emergency surgery
- Open aortic stent implant or thoracic endovascular aortic repair (TEVAR)
Acute myocardial infarction
A type of acute coronary syndrome, which is either STEMI, NSTEMI, or unstable angina (UA). Risk factors: Hypertension, atherosclerosis.
Patient presents with angina, dyspnoea, anxiety.
ECG may show ST elevation or new onset LBBB (STEMI), or ST depression, T-wave inversion, or normal (NSTEMI or UA). Troponins are elevated (STEMI or NSTEMI), or normal (UA).
Treatment:
- Initial
- ABC
- Morphine – only if pain is refractory to nitro
- Morphine worsens outcome and should not be used unless necessary
- Oxygen – only if saturation < 90%
- Nitroglycerine sublingual – only if pain does not improve and there is no RV infarct
- Aspirin – 300 mg chewed all cases
- Beta blocker – metoprolol oral
- Statin – atorvastatin oral
- P2Y inhibitor (clopidogrel/prasugrel)
- Revascularization
- PCI if available within 120 minutes
- Thrombolysis otherwise
Pneumothorax
PTX is air in the pleural space, causing lung collapse. If air is allowed to enter but not leave the pleural space, tension PTX occurs, which causes haemodynamic collapse. Risk factors:
- Tall, thin, young men
- Rupture of bullae (from weed smoking or emphysema)
- Trauma
- Iatrogenic
Patient presents with sudden, severe, stabbing ipsilateral chest pain and dyspnoea. Physical examination shows decreased breath sounds and hyperresonance on percussion on the affected side. Tension PTX also causes features of haemodynamic instability, like tachycardia, hypotension, distended neck veins, cyanosis.
Chest x-ray or eFAST can show PTX, but tension PTX is a clinical diagnosis and we don’t do imaging for it.
Treatment:
- Supplemental oxygen
- Small asymptomatic PTX -> observation
- Large or symptomatic PTX -> chest drain with suction
- Tension PTX -> immediate thoracocentesis
- 3rd ICS, mid-clavicular line or 5th ICS at mid-axillary line
- Later, chest drain
3. The acute management of massive pulmonary embolism
Definition
Massive pulmonary embolism refers to PE which causes obstructive shock and haemodynamic instability, usually because of a saddle-thrombus. Risk factors:
- Hereditary pro-coagulant mutation (Leiden, AT III def, etc.)
- Immobility
- Post-surgery
- Smoking
- Obesity
- Etc.
Clinical features
No specific symptoms. Pleuritic chest pain, dyspnoea, tachypnoea, haemoptysis, jugular vein distension. Unilateral painful leg oedema due to DVT. Massive PE gives syncope, obstructive shock.
Evaluation
Wells score for determining the probability of PE:
| Criteria | Points |
| Symptoms of DVT | 3 |
| PE more likely than other diagnoses | 3 |
| Previous PE/DVT | 1,5 |
| Tachycardia | 1,5 |
| Recent surgery or immobilization | 1,5 |
| Haemoptysis | 1 |
| Malignancy | 1 |
If score > 4, PE is likely.
Negative D-dimer rules out PE. ABG can show hypocapnia, hypoxia. Troponins, NT-proBNP can be positive. ECG can show abnormal, non-specific signs, like anterior T-wave inversion.
CT pulmonary angiography is gold standard for diagnosis. V/Q scan is second choice.
Acute management
- ABC
- Supportive treatment
- Oxygen supplementation or ventilation
- Fluid resuscitation
- Massive PE -> thrombolysis or embolectomy
- Non-massive PE -> anticoagulation or IVC filter
- Anticoagulation with LMWH or UFH
- IVC filter if AC is contraindicated
4. Management of acute rhythm disturbances
Etiology
- Myocardial infarction or CAD
- Electrolyte disorders with K+, Mg2+, Ca2+
- Drug intoxication (TCA, digoxin, beta blocker, CCB)
- Myocarditis
- Long QT syndrome
Why are they important?
Arrhythmias are important for three reasons:
- They cause suboptimal contraction, reducing cardiac output
- They may predispose to thromboembolism
- The arrhythmia may worsen, causing cardiac arrest
Evaluation
- Is there electrical activity at all?
- How much is the ventricular rate?
- Is the rhythm regular or irregular?
- Evaluation of QRS – is it wide or narrow? – limit is 120 ms
- Is there atrial activity?
- Is there a connection between P waves and QRS complexes?
- Is the patient clinically and haemodynamically stable or unstable?
- Are signs of peri-arrest (OMG signs – topic 15) present?
Bradyarrhythmias
- Mobitz type II 2nd degree AV block
- 3rd degree (complete) AV block
- Sick sinus syndrome
- Carotid sinus hypersensitivity
Management:
- ABC
- Atropine 0,5 mg – first choice
- If atropine does not give satisfactory response, either:
- Repeat atropine up to max 3 mg
- Give isoprenaline or adrenaline
- Begin transcutaneous pacing
Tachyarrhythmias (with pulse)
- Narrow-complex tachycardia
- Regular
- Sinus tachycardia
- Atrial flutter
- AVRT, AVNRT
- Irregular
- Atrial fibrillation
- Regular
- Broad-complex tachycardia
- Regular
- (Monomorphic) ventricular tachycardia
- Supraventricular tachycardia with BBB
- Irregular
- Atrial fibrillation with BBB
- Polymorphic ventricular tachycardia (torsade)
- Regular
Management:
- ABC
- Unstable tachyarrhythmia -> electrical cardioversion (synchronized mode)
- Unstable = chest pain (ischaemia), shock, syncope, heart failure
- Up to 3 attempts, then amiodarone 300 mg
- Stable tachyarrhythmia
- Regular narrow-complex tachycardia -> vagal manoeuvres, adenosine
- Irregular narrow-complex tachycardia -> beta blockers, diltiazem, digoxin
- Regular broad-complex tachycardia -> amiodarone, adenosine
- Irregular broad-complex tachycardia -> magnesium (for torsade)
Arrhythmias without pulse
- Shockable
- Ventricular fibrillation
- Pulseless ventricular tachycardia
- Non-shockable
- Asystole
- Pulseless electrical activity
Management:
- CPR
- Shockable:
- 200 J asynchronous defibrillation
- Reassess rhythm every 2 minutes
- Adrenaline 1 mg every 3 – 5 minutes
- Amiodarone 300 mg after 3 shocks, 150 mg after 5 shocks
- Non-shockable
- Adrenaline 1 mg immediately and every 3 – 5 minutes
- Reassess rhythm every 2 minutes
5. Hemodynamic monitoring (arterial line, central line insertion, invasive hemodynamic monitoring)
Arterial line
= catheter inside an artery, often radial, ulnar, axillary, brachial, etc. Very often used in the ICU.
Can be used for arterial blood gas (ABG) measurement and invasive arterial blood pressure monitoring (IABP), which has the advantage of being continuous.
Before insertion into radial or ulnar, the Allen test must be performed. The catheter is inserted with the Seldinger technique (over a guidewire).
Central line
= catheter into a central vein, often the jugular, subclavian, femoral vein.
Can be used for easy blood sampling, administering fluids and medications, and measuring certain parameters:
- SvO2 = mixed venous oxygen saturation
- The oxygen saturation of the blood in the RV/pulmonary artery
- Normally ~75%
- ScvO2 = central venous oxygen saturation
- The oxygen saturation of the blood in the SVC
- Normally 2 – 3% lower than SvO2
- CVP = central venous pressure
- The pressure of venous blood in the vena cava. Corresponds to the right atrial pressure and the preload
- Normally 5 – 10 cmH2O
Also inserted with Seldinger technique, often ultrasound guided.
Invasive haemodynamic monitoring
Nowadays we use PiCCO (Pulse indicator Continuous Cardiac Output). It has replaced the classic Swan-Ganz or pulmonary arterial catheter. PiCCO is used in haemodynamically unstable patients. It uses transpulmonary thermodilution and pulse contour analysis to calculate its parameters.
PiCCO requires both a central venous line and an arterial line, often in the femoral artery. It gives us the following parameters:
- Indicators of cardiovascular status
- Cardiac output (CO)
- Indicators of filling pressures
- Central venous pressure (CVP)
- Pulmonary capillary wedge pressure (PCWP)
- Indicators of cardiac preload
- Global End-Diastolic volume (GEDV)
- Intrathoracic blood volume (ITBV)
- Indicators of fluid responsiveness – “will fluids increase CO?”
- Stroke volume variation (SVV)
- Pulse pressure variation (PPV)
- Indicators of afterload
- Systemic vascular resistance (SVR)
- Indicators of cardiac contractility
- Global ejection fraction (GEF)
- Pressure velocity increase (dPmx)
- Cardiac function index (CFI)
- Indicators of pulmonary oedema
- Extravascular lung water (EVLW) – “how much water is in the lungs?”
- Pulmonary vascular permeability (PVP) – “why is there pulm. oedema?”
6. Acute management of fluid imbalance
Types of fluid
“Pure water” infusions are not actually pure water, but 5% dextrose (glucose) in water (D5W) infusions. Pure water has 0 osmolarity and so would cause haemolysis, but D5W has near-physiological osmolarity (250 mOsm/L). The glucose is rapidly metabolised, yielding pure water. D5W is used in pure water deficit, like dehydration or in patients unable to drink.
Crystalloid solutions are those which contain electrolytes. No crystalloid infusion contains the exact same electrolyte composition as plasma, but those which are similar are called “balanced” crystalloids while those which aren’t are called “unbalanced” crystalloids. Crystalloids are the most widely used infusions and can be used to replace any fluid loss. 1 L of blood loss can be replaced by 4 L of crystalloids.
“Physiological” saline/0,9% NaCl solution is widely known and widely used. However, it’s an unbalanced solution because it contains more sodium and chloride than plasma, and none of the other electrolytes. As such, it should not be used to replace lots of fluid, because the high chloride content can cause hyperchloraemic metabolic acidosis.
Balanced solutions like Ringer-lactate, sterofundin and isolyte are more similar to plasma and are better suited for replacing large volumes. However, Ringer-lactate contains less sodium than plasma and therefore can cause hyponatraemia if given in large doses.
For calculating maintenance fluid requirement per hour of a patient, regardless of age, do this: (4:2:1 rule)
- 4 mL of fluid per kg in 0 – 10 kg
- + 2 mL of fluid per kg in 10 – 20 kg
- + 1 mL of fluid per kg above 20 kg
Colloid solutions contain large solute molecules which can not leave the blood vessels. In theory this allows the solution to stay in the vessels rather than entering the interstitial and intracellular space, contributing more to circulating volume. These solutions contain albumin, gelatine, dextrans, etc. Some argue that colloids should be used in shock, but there’s little evidence that colloids are better than crystalloids.
Management of fluid imbalance
- Exsiccosis -> D5W
- Emergency -> physiological saline
- Long-term infusion -> balanced crystalloid
- Haemorrhage -> blood + crystalloid. Coagulation factors, thrombocytes if necessary
- Hypoproteinaemia -> albumin
- Severe hyponatraemia -> hypertonic saline
- Cerebral oedema -> hypertonic saline
- Shock -> crystalloid (and/or colloids?)
7. Acid-base disorders and management
Metabolic acidosis
pH < 7,35 and HCO3– < 21 mM. Negative base excess (BE). Due to compensation, hyperventilation (Kussmaul) occurs, which decreases pCO2 < 35 mmHg.
- High anion gap metabolic acidosis
- Diabetic ketoacidosis
- Lactic acidosis
- Renal failure
- Ingested toxins (methanol, ethylene glycol)
- Normal anion gap (hyperchloraemic) metabolic acidosis
- Diarrhoea
- Excessive physiological saline infusion
Clinical features: Kussmaul breathing, tachycardia, arrhythmia, decreased myocardial contractility, altered mental status
Treatment:
- Fluid resuscitation
- Sodium bicarbonate – if severe (< 7,0)
- Renal replacement therapy
- Treat underlying cause
- Ethylene glycol intoxication -> ethanol or fomepizole IV
Metabolic alkalosis
pH > 7,45 and HCO3– > 33 mM. Positive base excess (BE).
- Due to H+-loss
- Vomiting
- Loop or thiazide diuretics
- Hyperaldosteronism
- Due to alkali load
- Ingestion of alkali
Clinical features: Arrhythmia, altered mental status, tetany
Treatment:
- Fluid resuscitation
- Treat underlying cause
Respiratory acidosis
pH < 7,35 and pCO2 > 45 mmHg. Due to chronic compensation, HCO3– will elevate.
- Respiratory depression (trauma, depressant drugs)
- Respiratory muscle weakness (GBS, myasthenia gravis, muscle relaxants)
- COPD
- Airway obstruction
Treatment:
- Ventilation (invasive or non-invasive)
- Treat underlying cause
Respiratory alkalosis
pH > 7,45 and pCO2 < 35 mmHg. Due to chronic compensation, HCO3– will decrease.
- Pulmonary embolism
- Hyperventilation due to anxiety, pain
- Excessive mechanical ventilation
- CNS infection
Treatment:
- Breathing into a paper bag
- Treat underlying cause
8. Infection and infection control on the ICU
Although intensive care units (ICUs) account for fewer than 10% of total beds in most hospitals, more than 20% of all nosocomial infections are acquired in ICUs. ICU-acquired infections account for substantial morbidity, mortality, and expense. Most bacterial infections that occur on the ICU have some sort of anti-microbial resistance.
Most common infections:
- Catheter-associated UTI
- E. coli
- Ventilator-associated pneumonia (VAP) – occurs > 48 hours after initiation of invasive ventilation
- Gram-negatives (pseudomonas, E. coli)
- S. aureus
- Catheter-related bloodstream infection (CRBSI)
- Staphylococci
Most problematic pathogens:
- Vancomycin-resistant enterococci (VRE)
- MRSA
- Pseudomonas aeruginosa
- Multidrug-resistant gram-negative bacteria
Risk factors:
- Patients in poor condition
- Invasive interventions and devices
- Older age
- Comorbidities
- Long duration of hospitalization
- Antibiotic use
Prevention:
- Proper hygienic measures
- Ensure sterility during procedures
- Avoid invasive procedures when possible
- Discontinue indwelling sources of infection when possible
- Education of staff
- Etc.
9. Basic management of sepsis, severe sepsis, and septic shock
Definition
Sepsis is as a life-threatening organ dysfunction caused by a dysregulated host response to infection. It’s defined based as a clinical suspicion of infection + a SOFA score of 2 or more.
Septic shock is a subset of sepsis in which circulatory, cellular, and metabolic abnormalities are profound enough to substantially increase mortality. It’s defined as sepsis plus both of the following:
- Persisting hypotension requiring vasopressors to maintain MAP > 65 mmHg
- Persisting lactate > 2 mM despite volume resuscitation
SOFA (sequential organ failure assessment) score is used to evaluate the function of the following organ systems:
- Respiratory system (PaO2/FiO2)
- Coagulation (thrombocytes)
- Liver (bilirubin)
- Cardiovascular system (MAP)
- CNS (GCS)
- Kidney (creatinine)
Depending on the degree of abnormality for each organ system, a score of 0 – 4 is given. A score of 2 is required for the diagnosis of sepsis.
A derivative score, quick SOFA (qSOFA), is used to quickly screen for the need to measure a proper SOFA score. One point is given for each of the following:
- Respiratory rate > 22/min
- Altered mental status
- Systolic BP < 100 mmHg
A score of 2 or 3 means that a proper SOFA should be evaluated.
Management
Management according to the 2016 surviving sepsis campaign (SSC). The therapeutic goals are:
- Central venous pressure 8 – 12 mmHg
- Mean arterial pressure > 65 mmHg
- Urine output > 0,5 mL/bwkg/hour
- SvcO2 > 70%
Specific steps of management
- Within 1 hour
- Two sets of blood cultures
- Measure lactate and the other components of SOFA
- Broad-spectrum antibiotics
- Initial resuscitation with crystalloid, at least 30 mL/kg in the first 3 hours
- If insufficient, use vasopressor (norepinephrine is first choice)
- If insufficient, use hydrocortisone
- As soon as possible and if necessary
- Source control in case of localized infection
- Drainage of infection, necrectomy, etc
- Blood products
- RBCs in case of Hb < 7,0 g/dL or acute bleeding
- Thrombocytes in case of thrombocytopaenia
- Glucose control
- Early enteral feeding
- Sedation – not too deep. Preferably in an arousable state
- Lung-protective ventilation
- Urinary catheterisation
- Bicarbonate in case of severe metabolic acidosis
- Organ support
- ECMO
- Renal replacement therapy
- Source control in case of localized infection
- Prophylaxis for
- Venous thromboembolism (UFH or LMWH)
- Stress ulcer (PPI)
10. Multiple organ failure
Multiple organ dysfunction syndrome (MODS) or multiple organ failure (MOF) is defined as the acute insufficiency of 2 or more organ systems, requiring intervention to maintain homeostasis. It has high mortality, and the mortality increases with the amount of failing organ systems. It is mostly seen as an end-stage of sepsis or other severe systemic inflammation like acute pancreatitis.
The SOFA score is used to evaluate organ function to diagnose MOF.
11. ARDS, definition and basic ventilatory management
Definition
Acute respiratory distress syndrome (“ARDS”) is a life-threatening inflammation with oedema in the lungs which leads to severe respiratory failure. It occurs in approx. 10% of ICU patients, and there is a 50% mortality rate.
Etiology:
- Sepsis (most common)
- Pneumonia
- Aspiration of gastric content
- Shock
- Pancreatitis
- Major trauma
Clinical features:
- Dyspnoea, restlessness, anxiety
- Altered mental status
- Cyanosis
Diagnosis by the Berlin criteria. All four must be met:
- Respiratory failure within one week of a known cause of ARDS
- Bilateral opacities on x-ray or CT
- Hypoxaemia (decreased PaO2/FiO2)
- The degree of hypoxaemia determines whether it’s mild, moderate, or severe ARDS
- Heart failure or fluid overload does not account for the respiratory failure
Management
- Treat the underlying condition
- Supportive oxygen therapy (non-invasive ventilation)
- Non-invasive ventilation may be tried in mild-moderate ARDS, but 50% of patients require intubation later anyway
- Intubation and invasive ventilation should not be delayed
- Lung-protective ventilation (invasive ventilation)
- Lung-protective ventilation refers to changing the settings of the ventilator to protect the lung from barotrauma and volutrauma during invasive ventilation
- This means low tidal volume, low plateau pressure, and high positive end-expiratory pressure (PEEP)
- These settings cause hypercapnia but that’s not a problem and we allow it. This is called permissive hypercapnia
- Neuromuscular blockers if severe
- Cisatracurium
- Must be used early
- Prone positioning if moderate or severe
- Fluid overload should be avoided
- ECMO – if all other methods of oxygenation fail
- If due to COVID-19: Dexamethasone and tocilizumab
12. Indications and basis of mechanical ventilation
Normal values:
- O2 pressure in artery (PaO2) ~ 100 mmHg
- O2 pressure in vein (PvO2) ~ 40 mmHg
- Alveolo-arterial gradient (PA-aO2) < 20 mmHg
Variable performance oxygen therapy devices
The oxygen delivery of these devices depends on the patient’s own breathing. These devices enrich the air with oxygen during inspiration. There are three types, the nasal cannula, 50% mask, and 100% mask.
The nasal cannula gives an FiO2 of 30%, and it can give 2 – 6 L/min of oxygen. They’re comfortable and cheap.
The 50% face mask gives an FiO2 of 50%, and it can give 5 – 10 L/min of oxygen. However, CO2 may accumulate in the dead space of the mask, causing CO2 retention.
The 100% face mask gives an FiO2 of 100% in theory, but 80% in practice. It can give 5 – 15 L/min of oxygen. It has a one-way valve which prevents CO2 trapping. Neither type of face mask humidify the air, which is a disadvantage.
Fixed performance oxygen therapy devices
These devices deliver a fixed amount of oxygen and do not depend on the patient’s breathing. They can provide an O2 flow rate of 30 – 60 L/min. These include the Venturi mask and high flow oxygen therapy (HFOT).
Mechanical ventilation
Mechanical ventilation refers to the use of a ventilator to assist or replace the patient’s breathing. It is necessary when the O2 uptake or CO2 elimination is insufficient (respiratory failure), when the respiratory muscle power is reduced, and when there is severe circulatory failure. Mechanical ventilation can be non-invasive (NIV) or invasive.
Indications:
- PaO2 < 60 mmHg at room air
- PaCO2 > 50 mmHg
- Except in COPD, who may have compensated hypercapnia
- PaO2/FiO2 < 300 mmHg
- pH < 7,25 (respiratory acidosis)
Non-invasive ventilation can be used with a nasal mask, face mask, a full face mask, or a helmet. NIV does not protect the airways, and so the patient must be cooperative and protect their own airways. It can also not be used in severe gas exchange disorder. If non-invasive ventilation is not successful in improving the condition of the patient, invasive ventilation is required.
Invasive ventilation requires intubation or, if ventilation is required long-term, a tracheostomy. It is more effective than NIV, but it’s more invasive and can therefore increase the risk for VAP. It is difficult to wean people off invasive ventilation and back on spontaneous ventilation. It may take weeks.
Ventilator-induced lung injury (VILI) refers to injury of the lung due to the ventilator. It’s usually avoidable when using appropriate and proper settings and parameters. This can occur in the form of barotrauma (excessive pressure causes rupture of alveoli), volutrauma (excessive volume causes overdistension of alveoli), biotrauma (release of inflammatory mediators), and atelectrauma (repeated opening and closing of alveoli).
The ventilator can either be in pressure control mode or volume control mode. In volume control mode, the operator sets a tidal volume for the patient, and the machine selects the pressure required to achieve that tidal volume. Pressure control mode is the opposite. There are advantages and disadvantages of each mode.
A ventilator can also provide positive end-expiratory pressure (PEEP), where it keeps a positive pressure in the airways after the expiration to prevent alveolar collapse (atelectasis) during expiration. A small amount (3 – 5 cmH2O) of PEEP is used in most ventilated patients. This has other advantages as well but also some disadvantages:
- Advantages
- Prevents atelectasis
- Increases functional residual capacity (FRC)
- Increases gas exchange area
- Increases compliance
- Decreases preload
- Decreases afterload
- Decreases V/Q mismatch
- Decreases work of breathing
- Disadvantages
- Decreased CO2 elimination
- May decrease cardiac output in right heart failure or hypovolaemia
- Increases ICP
- Increases intrathoracic pressure -> may cause PTX
13. Management of acute respiratory illnesses (acute exacerbation of COPD, asthma)
Acute exacerbation of COPD
Acute exacerbations of COPD are usually caused by viral respiratory infections, bacterial infections, pollution, and stress. It may be life-threatening and so rapid assessment of the severity is important.
The cardinal symptoms of acute exacerbations are worsening dyspnoea, worsening cough, increased volume and/or purulence of sputum. In severe cases, respiratory failure may occur. If respiratory failure occurs, or if symptoms are severe, or if the patient has serous comorbidities, or if out-patient treatment has failed to improve symptoms, hospitalization is needed.
The diagnosis is based on clinical symptoms. If there are severe symptoms, ABG is used to assess the level of severity.
Most cases can be treated with supplemental oxygen and bronchodilators, with antibiotics if infectious. Admission to the ICU is indicated if:
- If conservative therapy doesn’t work
- PaO2 < 40 mmHg
- pH < 7,25
- Haemodynamic instability
In the ICU, non-invasive ventilation is used in most cases. Invasive ventilation is used in very severe cases.
Asthma exacerbation
Asthma exacerbations are characterized by episodes of progressive increase in shortness of breath, cough, wheezing or chest tightness. Severe exacerbations are potentially life-threatening, and treatment requires close supervision. It usually occurs due to exposure to factors like exercise, air pollution, allergens, or infections.
Patients present with dyspnoea, accessory breathing muscles, chest tightness. They can usually not complete sentences in one breath. They usually have wheezing on auscultation, but the chest can also be silent on auscultation, in which case the situation is life threatening.
ABG shows hypocapnia with normoxaemia or hypoxaemia.
Most cases can be treated with supplemental oxygen, bronchodilators, and systemic steroids. Magnesium sulphate can be given as a smooth muscle relaxant. Admission to the ICU is indicated if:
- If conservative therapy doesn’t work
- PaO2 < 60 mmHg
- History of near-fatal asthma
- Peak expiratory flow rate < 30%
In the ICU, bronchodilators can be repeated as IV. NIV may be used, but invasive mechanical ventilation is more used. It’s important to look for and treat the underlying problem.
14. Monitoring and treatment of acute renal failure
Acute kidney injury occurs frequently in the ICU. It’s defined as an acute decline in kidney function causing an increase in serum creatinine, often with decreased urine output. Oliguria is defined as < 0,5 mL/bwkg/hour. Several classifications and criteria for AKI exist, like RIFLE, AKIN, and KDIGO. They classify AKI according to the serum creatinine and urine output.
AKI can be caused by pre-renal, renal, or post-renal causes. Prerenal causes and acute tubular necrosis (ATN) are the most common causes.
Prevention of AKI
NSAIDs and RAAS inhibitors can precipitate AKI in elderly and people with renal artery stenosis or volume depletion, so they must be used carefully.
IV contrast can cause AKI, especially in people with CKD and volume depletion.
Crush injuries and rhabdomyolysis can cause AKI. They must be treated with aggressive fluid resuscitation and bicarbonate to prevent myoglobin precipitation.
Treatment of AKI
Treatment involves treating the underlying cause. Furosemide may be used to increase urine output, but only if the patient has proper volume status.
Renal replacement therapy (RRT) is indicated if:
- Oliguria or anuria
- Severe hyperkalaemia
- Severe acidosis
- Uraemic signs
- Drug overdose with dialysable drug
There are multiple types of RRT, the most important of which are haemodialysis and haemofiltration.
15. Early warning signs and peri-arrest period
Peri-arrest
The peri-arrest state is a condition presenting just before cardiac arrest, indicating the need for immediate intervention and/or preparation for CPR. It’s important to recognize so that CPR can be initiated early. For every minute CPR is not initiated after cardiac arrest, survival decreases by 7-10%. A peri-arrest state occurs in 80% of cases.
The “OMG” signs of peri-arrest:
- Respiratory rate > 30/min or < 8/min
- Heart rate > 140/min or < 40/min
- Systolic BP > 220 mmHg or < 90 mmHg
- GCS decrease by > 2
- Imminent airway obstruction (decrease in SpO2)
Other important signs of peri-arrest:
- Seizure
- Asystolic periods (> 2 – 3 seconds)
- Acute significant bleeding
- Any unexplained deterioriation
Immediately perform ABCDE management. Secure airways and breathing, monitor and support circulation, check for disabilities and exposure/environment.
Early warning signs
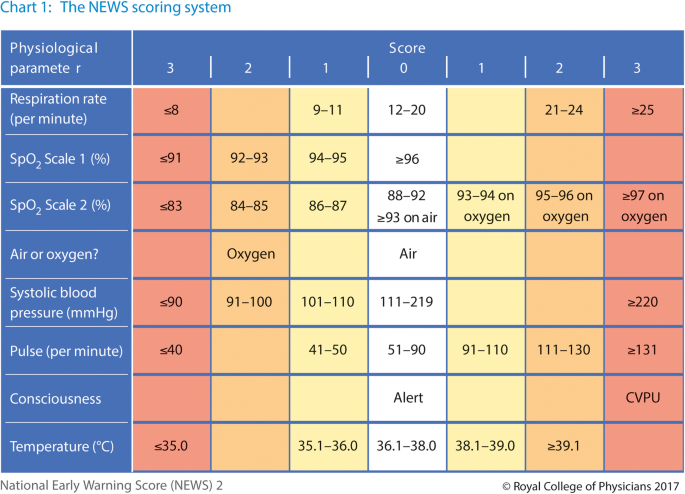
The (national) early warning score (NEWS or EWS) gives scores for various parameters and can be used to predict the possibility of cardiac arrest. A score of 5 or more means high possibility of cardiac arrest, and so a doctor should be informed and the patient should be closely monitored.
This score is usually measured in all inpatients at regular intervals. The intervals are shorter the higher the score.
16. Nutrition of the critically ill (types of nutrition and indications)
Malnutrition is a serious problem that increases the risk of morbidity and mortality and has high incidence amongst intensive care patients. Most of these patients (e.g. sepsis, severe burn) have hypermetabolism and increased protein catabolism. Nutrition support in the form of enteral or parenteral nutrition is used.
The indications are as follows:
- Critically ill patients who cannot eat sufficiently
- Inability to swallow (stroke, oesophageal cancer)
- Low GCS
- Severe anorexia
Early nutrition support is indicated for most critically ill patients, especially those with hypermetabolism.
Enteral feeding
Enteral feeding means delivery of nutrition directly into the GI tract either orally or through a tube into the stomach, duodenum, or jejunum. Except for the oral form, this requires placement of a feeding tube, like a nasogastric, orogastric, or nasojejunal tube, or an enterostomy. In the oral form, the patient drinks liquid formulas per os. Enteral feeding is preferred to parenteral feeding, because of the following advantages:
- Mucosal atrophy is prevented
- Metabolic complications occur less frequently
- Blood stream infections occur less frequently
- It is cheaper
Some contraindications include:
- Mechanical ileus
- Acute abdomen
- Recent GI anastomosis
- Intestinal ischaemia
Special formulations exist for special needs. For fluid-restricted patients, a more concentrated formulation exists. For patients with renal failure, an electrolyte-restricted formulation exists.
Enteral feeding is usually given in 10 – 30 mL/hour. The bed should be at an angle to prevent aspiration.
Parenteral feeding
Parenteral feeding means delivery of nutrition directly into a peripheral or central vein. This has more complications and is used as a last resort. Two forms exist, partial parenteral feeding (where a combination of enteral and parenteral feeding is used), and total parenteral feeding.
17. Mental disorders, drug overdosed patients (the unconscious patient and toxins)
Disorders of consciousness
The disorders of consciousness are, in Hungarian literature, usually separated into hypnoid and non-hypnoid types. The hypnoid ones are the most important. They are:
- Somnolence – patient is sleepy but can be aroused by voice
- Sopor – patient is unconscious and can only be aroused by pain
- Coma – patient is unconscious and cannot be aroused
Consciousness is usually evaluated by the Glasgow coma scale, which scores the consciousness from 3 – 15, based on the motor response, verbal response, and eye response to commands or stimuli. A score of 8 or less is an indication for intubation, as the person is no longer able to protect their airway.
It can also be evaluated by the much simpler AVPU scale, where the patient is marked as either:
- A – alert
- V – responds to verbal stimulus
- P – responds to pain
- U – unresponsive
The possible causes of disordered consciousness are endless but can be remembered by mnemonics:
- ATOMIC
- Alcohol, trauma, overdose, metabolic, infection, CO2
- I WATCH DEATH
- Infection, withdrawal, acute metabolic, trauma, CNS pathology, hypoxia, deficiencies, endocrine, acute vascular, toxins, heavy metal poisoning
Delirium
Delirium is an acute state of confusion with fluctuating mental status, disordered attention, and disorganised thinking. The patient may be hypoactive or hyperactive. It’s clinically relevant because it increases mortality, prolongs the hospital stay, and increases reintubation rate. It usually occurs during acute illness and is therefore common in the ICU. It’s especially common in the ICU because many of the patients are elderly and because there is a lot happening at night in the ICU, so sleep withdrawal is common.
Patients may be disoriented, have disturbed sleep-wake cycles, hallucinate, have delusions, etc.
The patients can be screened with the intensive care delirium screening checklist (ICDSC) or confusion assessment method in the ICU (CAM-ICU).
The treatment involves treating the underlying cause. Haloperidol is the first choice if calming down the patient is necessary.
Drug intoxication
In a patient with suspected drug intoxication, the following general steps are necessary:
- Ensuring stability (ABC)
- Give oxygen
- Apply monitoring
- Obtain venous access
- Assess GCS and intubate if < 8
- ABG
- Decontamination
Toxicology does not give an immediate answer, so the suspected toxin should be identified based on lab results, history, and clinical features.
In any kind of intoxication, the following are options:
- Gastric lavage or activated charcoal – not effective in all intoxications, only effective in ingestion was recent
- Loop diuretics + fluids – to increase renal excretion
- Renal replacement therapy – haemodialysis or haemofiltration
Sympathomimetic (amphetamine, MDMA, cocaine) overdose presents with mydriasis, hyperthermia, tachyarrhythmia, hypertension, seizures, altered mental status. There is no specific treatment.
Opiate overdose presents with respiratory depression, pinpoint pupils, and altered mental status. Naloxone is the specific antidote.
On my exam I said shallow breathing, but the examiner said that it’s rather deep breathing but bradypnoea. That doesn’t match up with other sources, though.
Benzodiazepine overdose presents with weak pulse, respiratory depression, and altered mental status. Flumazenil is the specific antidote, but it has a short duration of action and is therefore used for diagnosis rather than treatment.
18. Critical care of polytrauma victims
Polytrauma is an epidemic, and it’s the leading cause of death for people under 40. It’s most simply defined as the presence of multiple injuries when the effects of these injuries are multiplied and more complicated to treat than the sum of the isolated injuries. Shock, SIRS, and MODS can occur. Often, saving all the functions of the victim is impossible and so compromises must be made. It’s important to keep in mind the saying “life before limb”, meaning that one should amputate a limb if it could save the life.
Pre-hospital care
The goal of pre-hospital care is to stabilise the patient sufficiently for the transport to the hospital.
ABCs are the priority on scene, after ensuring the safety of the environment first. The patient should be stabilised with pain management and fluid resuscitation if needed. It’s important to not give fluids too aggressively, as that will cause haemodilution, which dilutes the clotting factors, predisposing to more bleeding. Hypothermia should be prevented. Pain management decreases O2 demand of the body.
During transport, it’s essential that the patient is adequately fixated to prevent secondary injury, especially of the cervical spine. Fixation can be accomplished with boards, splints, cervical collar, etc.
Hospital care
In hospital, the ABCs are repeated immediately to further stabilise the patient for surgery. The patient should be intubated if there are indications for it. Give blood products if the blood loss is severe (> 30%). eFAST to check for internal bleeding. CT if the patient is stable enough for it. Administration of O2 is esssential.
When the patient is stable enough, they’re taken to the operating room, where the first priority of the trauma surgeons is to treat life-threatening injuries, most notably intracranial, intrathoracic, or intraabdominal bleedings. This is the first operative phase, during which only those injuries which make stabilisation of the patient’s vitals impossible should be treated. Surgical treatment of less urgent injuries, like injuries threatening a limb, occurs later.
Following this, the first stabilising phase starts, in which one uses stabilising measures and monitoring to stabilise the patient’s vitals, including urine output, acid-base status, gas exchange, and blood volume.
After only a few hours of stabilising, the second operative phase starts. Here, potential life-threatening injuries, injuries that may threaten a limb, and other injuries which make care of the patient difficult, must be treated. One should not attempt to save a limb if it may risk the patient’s life.
Then, the second stabilising phase starts, where similar goals as the first stabilising phase are important. This phase ends when the patient no longer requires organ support and intensive therapy. The patient continuously monitored in the ICU for late-onset complications like sepsis, SIRS, or MODS.
After the patient no longer requires intensive care, the third operative phase starts, where non-life threatening injuries are treated.
Damage control surgery
When possible, polytrauma patients should have definitive surgery (surgery aimed to permanently fix the problem) as early as possible as this improves survival. However, on the other hand, prolonged surgery on haemodynamically and vitally unstable patients decreases survival. For these patients, damage control surgery is the alternative.
Damage control surgery refers to surgery which is necessary to stabilise (but not definitively treat) life-threatening conditions in the acute phase of polytrauma. In case of severe fractures, for example, this could be to fixate the fractures with minimally invasive external frames as a temporary solution until the patient is more stable for definitive fracture fixation after the patient has stabilised, usually a few days later. The core principles of damage control surgery are:
- Control the source of bleeding as early as possible
- Treatment of coagulopathy
- Acidosis prevention/correction
- Hypothermia prevention/treatment
- Cause minimal iatrogenic injury (haemodilution by administering too much fluid)
Damage control surgery is indicated in:
- Pelvic and long bone fractures which cause haemodynamic instability
- Compartment syndrome
- Large soft tissue defects
- Occipito-cervical dissociation
- Ustable spinal fracture
- Penetrating injury of the chest or abdomen
Damage control resuscitation
Polytrauma patients are prone to developing trauma-induced coagulopathy (TIC), which worsens haemorrhage, something polytrauma patients are usually already at risk of. Damage control resuscitation refers to those actions aimed at achieving haemostasis early and rapidly, to prevent TIC. Damage control resuscitation involves:
- Massive transfusion protocol (in which one transfuses 10 units of packed RBCs, platelets, and fresh frozen plasma in a 1:1:1 ratio)
- Permissive hypotension (maintaining systolic BP < 90 mmHg to decrease bleeding)
- Tranexamic acid
Indications for damage control resuscitation include sign of haemorrhagic shock and positive eFAST.
19. Critical care after central nervous system injury, treatment of elevated intracranial pressure
Traumatic brain injury
Traumatic brain injury (TBI) is a structural or physiological disruption of the brain due to a head injury. It’s a leading cause of death and disability in young adults. It can be open or closed depending on whether the skull is intact.
Primary brain injury occurs at the time of the head injury, while secondary brain injury results from physiological changes which follow the head injury, for example cerebral oedema or disruption of the BBB.
A concussion is a mild TBI with no visible structural damage to the brain, but with symptoms of TBI. A contusion is a focal intraparenchymal haemorrhage of the brain.
The severity of TBI is graded based on the GCS:
- Mild TBI – GCS 13 – 15
- Moderate TBI – GCS 9 – 12
- Severe TBI – GCS < 9
TBI may also be classified according to whether the injury is focal or diffuse:
- Focal
- Contusion
- Epidural haemorrhage
- Bleeding from MMA
- Good prognosis after surgery
- Subdural haemorrhage
- Bleeding from bridging veins
- Poor prognosis
- Diffuse
- Diffuse axonal injury
- Diffuse vascular injury
Patients present with:
- Loss of consciousness
- Sleepiness
- Headache
- Signs of increased ICP (papilloedema, vomiting, LoC)
- Focal neurological signs
- Signs of skull fracture (racoon eyes, rhinorrhoea, otorrhoea)
- Anisocoria
It’s important to note that unconscious TBI patients are considered to have a spinal injury until proven otherwise. Cervical CT is mandatory in these patients to rule out spinal injury, and these patients must not have their head moved until then.
CT is mandatory in all TBI patients who are not at low risk for poor outcome (mild or no symptoms). In patients with severe TBI, ICP monitoring should be performed.
The goals of management of TBI are to prevent secondary injury, by providing proper blood flow, perfusion pressure, and oxygen supply.
It’s important to intubate in the case of severe TBI. Hypocapnia should be avoided as it decreases CBF. Supportive therapy should be initiated to ensure normoxia, normotension, normoglycaemia, and normovolaemia.
Treatment of increased intracerebral pressure
Normal ICP is < 10 – 15 mmHg. Therapeutic options:
- Head elevation
- CSF drainage
- Sedation
- Mannitol or hypertonic saline
- Hyperventilation
- Hypothermia
- Barbiturates (pentobarbital or propofol)
- Decompressive craniectomy
Steroids are not used.
Spinal injury
Spinal shock refers to transient loss of spinal cord function below the level of the injury. It may take weeks for function to return. Haemodynamic monitoring is important as it’s accompanied by hypotension, etc.
Intubation is indicated with GCS < 9 or if the injury is above C4. General supportive therapy otherwise.
20. Critical care of severely burned patients
Depths of burn
- 1st degree – only the epidermis is affected
- 2nd degree – epidermis and dermis are affected
- 2A – upper layers of dermis affected
- 2B – deeper layers of dermis affected
- 3rd degree – epidermis, dermis, and subcutis affected
- 4th degree – muscle, fat, fascia, bones affected
1st degree burns form no blisters, but the skin is oedematous, red and painful. It heals without treatment or scar formation. 2nd degree burns type 2A form vesicles and bullae, is red and painful. It heals with abnormal pigmentation but without scarring. Type 2B also form vesicles and bullae, is red and painful. It heals with scar formation.
3rd degree burns are not painful. The skin is necrotic with black or grey skin. It does not heal without intervention. 4th degree burns is unsalvageable and requires amputation.
Severity of burn
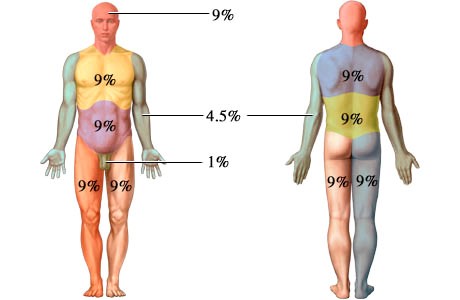
The extent of burn, the percentage of total body surface which is burnt (TBSA%), can be estimated by the Wallace rule of nines:
A severe burn is one who fulfils any of the following:
- Any burn complicated by major trauma or inhalation injury
- Chemical burns
- High voltage electrical burn
- Any burn >20% of the TBSA
Severe burns require intensive care in specialized burn centres. The patient must by haemodynamically monitored, often invasively (PiCCO). The urine output should be monitored and kept > 0,5 mL/kg/hour.
Potential complications of severe burns include:
- Shock
- Sepsis
- ARDS
- Infection
Management
In these patients, the following interventions are important:
- Fluid replacement
- Pain management
- Surgical treatment (debridement, necrectomy, skin graft, etc.)
- Washing every 2 days
- Stress ulcer prophylaxis
- Tetanus prophylaxis
The amount of fluid to replace can be calculated by the Parkland formula: 4 mL/kg of bw/TBSA%. Ringer-lactate is used. Half is given in the first 8 hours, half in the next 16 hours.
21. Cardio-pulmonary resuscitation
CPR is practiced during the semester, and a powerpoint presentation on the subject is provided by the department, so I won’t repeat those details here. By the end of the semester, you probably already know what needs to be known about this topic.
22. Definition and ethical aspects of brain-stem death
Brain death
In Hungary, brain death is defined in the law as “the irreversible cessation of all functions of the entire brain, including the brain stem”. To determine brain death, the following steps must be performed:
- There must be no exclusion criteria present, like:
- Poisoning, drug effects
- Coma due to shock, metabolic, endocrine conditions
- Hypothermia or hyperthermia
- CNS infection
- Confirmation of total absence of brain function
- No motor activity at all, neither spontaneous nor upon stimulation
- Absent brainstem reflexes (pupillary reflexes, corneal reflexes, gag reflex, etc.)
- Apnoea test – measures brainstem activity
- The patient is ventilated with 100% oxygen
- Then disconnected from the ventilator
- The patient is observed for spontaneous breathing
- After 10 minutes, an ABG is performed and the PaCO2 is measured
- If PaCO2 > 60 mmHg, there’s no activity of the brainstem respiratory centre
- (Spinal reflexes may still be present)
- Confirmation of the irreversibility of absent brain function during the observation period
- Repeat the above tests
- Imaging tests may be used to shorten the observation time (US, scintigraphy, angiography)
- Brain death must be confirmed by 3 independent specialist doctors who go through all the steps and who agree unanimously
The duration of the observation period depends on the injury. In case of primary brain injury, it is 12 hours, in case of secondary brain injury, it is 72 hours. For children < 3 years old, the period is longer.
Then, the organ coordination office must be called to inquire as to whether the patient is a donor or not. Contact relatives to give information. Their consent is needed in case of child donors.
The patient must be treated in the ICU while waiting for surgery, to maintain optimal perfusion of donor organs. There, the following are necessary:
- Fluid therapy
- Proper ventilation
- Replacement of pituitary hormones
- Temperature maintenance
Organ donation
In Hungary, the consent for organ donation is presumed, meaning that all persons are organ donors unless they have opted out of it during life. This is not the case for all countries, for example USA, Germany, and Norway rather have opt-in systems.
Hungary is part of Eurotransplant, an organization which organizes the allocation of donor organs in central/Eastern EU.
There are some contraindications to organ donation:
- Active infection (TBC, HBV, HCV)
- HIV
- Prion disease
- Malignancy
- Etc.
Examination questions in anaesthesia
1. Preoperative patient assessment and risk stratification, preparation for anaesthesia
Preoperative assessment
The preoperative assessment consists of the anaesthesiologist taking history, physical examination, medication history, and assessing comorbidities. It’s usually performed the day before planned surgery. Its purpose is to:
- Provide the patient with through information, which is important for informed consent
- Make the patients to ask questions and receive exhaustive answers
- Reduce the patient’s anxiety, introduce yourself
- Allow the anaesthesiologist to familiarize themselves with:
- The patient’s medical history, social history (smoking, drinking)
- The patient’s reactions to the previous anaesthesiologic procedures
- The patient’s symptoms
- Allow the anaesthesiologist to thoroughly assess the risks of anaesthesia
- To make suggestions for preoperative diagnostics and/or therapy
- To prepare the best possible anaesthesia plan
Sometimes, special tests are necessary, like pulmonary function test in case of severe respiratory disease (COPD, untreated asthma) or chest surgery. Tests are generally only performed if the result will affect the anaesthesia plan.
Preoperative risk stratification
ASA (American Society of Anaesthesiologists) categories are used to categorise patients according to their physical status:
- ASA 1 – healthy, non-smoking patient
- ASA 2 – patient with well-controlled disease with normal quality of life
- Overweight, well-treated hypertension
- ASA 3 – patient with disease which impacts normal functioning of patient
- Poorly treated DM, stable angina
- ASA 4 – unstable patient with disease is a constant threat to life
- AMI, stroke, etc.
- ASA 5 – patient unlikely to survive for > 24 hours without surgery
- Ruptured AAA, polytrauma
- ASA 6 – brain dead patient
Preparation for anaesthesia
Certain drugs must be stopped before surgery:
- Smoking – 24 hours before
- RAAS inhibitors – 1 day before
- Warfarin – INR should be < 1,3
- DOAC – 2 days before
- Metformin – 2 days before
- Antiplatelets – 7 days before
The patient must also stop eating the night before or at the latest 6 hours before, and no liquids at all in the last 2 – 4 hours.
In some cases, premedication is necessary:
- Benzodiazepines for anxiety
- Antiemetics for nausea
- Analgesics
- Antibiotic prophylaxis
In cases of low-risk (ASA 1 or 2) patients undergoing small surgical procedures, day-case surgery is an option. This decreases the hospital stay, is cheaper and has lower risk for nosocomial complications.
2. Airway maintenance, respiratory systems, anaesthetic machine
Airway maintenance and respiratory systems
There are several things which must be taken into account regarding airway maintenance:
- What is the patient’s body shape and airway anatomy? Will it make intubation difficult?
- Has the patient fasted?
- Are muscle relaxants needed?
- Will the surgery influence the anaesthesia somehow?
If the patient hasn’t fasted before surgery, for example during an emergency, the stomach is regarded as full, and rapid-sequence intubation (also called crash induction) and the Sellick-manoeuvre is needed.
- All equipment which will be and may be necessary are prepared
- The patient is preoxygenated with 100% oxygen
- The Sellick-manoeuvre is applied
- Anaesthesia and paralysis is induced simultaneously with succinylcholine and an IV anaesthetic
- The patient is rapidly intubated after step 3
- The Sellick-manoueuvre is stopped
Mask ventilation is avoided, as this would increase the risk of aspiration. The Sellick-manoeuvre involves placing pressure on the cricoid cartilage to compress the oesophagus and prevent aspiration.
An airway can be maintained with various tools:
- Face mask
- Nasopharyngeal (Wendl) tube
- Oropharyngeal (Guedel) tube
- Combitube
- Laryngeal mask
- I-gel tube
- Endotracheal tube (ET)
- Coniotomy – if mask ventilation and intubation fails (difficult airway)
The most frequently used airway is the ET tube. Signs of a successful intubation include:
- Direct visualisation of the tube passing between the vocal cords
- Auscultation of breathing sounds bilaterally
- Observation of chest movements
Anaesthetic machine
The primary function of the anaesthetic machine is to provide adequate amounts of oxygen and anaesthetic agent under controlled conditions, and to prevent the patient from inhaling their own exhaled air. It has three parts:
- Gas delivery system – delivers a mixture of inhaled anaesthetics, oxygen, and air
- CO2 – absorber containing soda lime
- Reservoir bag – provides a gas reservoir and allows us to evaluate the patient’s spontaneous ventilation
- Vaporizer – adds the anaesthetic
- Ventilator
- Monitor
The machine contains one-way valves which prevent the patient from inhaling their own exhaled air.
3. Principles of paediatric anaesthesia
Before anaesthesia
Repeated or lengthy general anaesthesia in children can negatively affect their brain development, but short courses have no negative effect. The risk is higher in preterms and newborns.
As always, a detailed history should be taken. It’s important to know about the vaccines, as 2 – 10 days must pass between vaccines and anaesthesia. Recent upper respiratory tract infection may also be a contraindication for anaesthesia.
It’s important to prepare the child and parents mentally, and to explain the procedure in detail, so they know what to expect. Written, informed consent is needed.
Children have more rapid gastric emptying than adults. They must stop eating 6 hours before and stop drinking 1 hour before. Breast milk must be stopped 3 hours before.
Premedication to prevent pain and anxiety is used. Oral, nasal, or rectal midazolam or dexmedetomidine is used, IV and IM are not. Non-pharmacological ways of decreasing pain and anxiety like games and distractions are also important.
It’s important to prepare the proper equipment for the age of the child before anaesthesia. Tubes, masks, laryngoscopes, dosages, etc. must be changed.
During anaesthesia
For children up to 6 – 8 year old, inhalational induction with mask is usually used to avoid needle puncture. Sevoflurane is usually used.
For older children, intravenous induction can be used. Propofol or barbiturates are used. Cannulation of peripheral veins in children can be difficult, even for trained personnel. The cannula must be fixed tightly so that it does not come out if the child wakes up.
Regional anaesthesia is used in paediatric anaesthesia as well, but unlike for adult anaesthesia, the patient can not be awake (i.e., general anaesthesia is required as well). After general anaesthesia has been applied, the regional anaesthesia is applied. This allows for the amount of general anaesthetic to be reduced.
Regional-only anaesthesia is used only in very minor procedures, or if general anaesthesia is risky for the child.
Post-operative
NSAIDs or paracetamol are given as premedication, with the intention to prevent postoperative pain. Regional anaesthesia given during anaesthesia is also very good at preventing postoperative pain for 6 – 12 hours. After major surgeries, only opioid analgesics are sufficient for pain management. Children as young as 8 years can operate a patient controlled analgesia (PCA) pump effectively.
Young children are less able to express pain, so we should use other parameters to evaluate how much pain they’re in. Elevated heart rate, respiratory rate, and blood pressure can indicate pain, but the most important changes are the behavioural changes in the child, e.g. how the child is moving, the facial expressions, crying, sleep:awake ratio, etc.
Older children can use pain scales like the visual analogue scale (VAS) to express their degree of pain.
4. Pharmacology of inhalational anaesthetics and intravenous anaesthetics
Inhaled anaesthetics
These are lipid-soluble, hydrophobic drugs. The more lipid-soluble the drug, the:
- Higher the potency (the lower the minimal alveolar concentration (MAC))
- Slower the induction of anaesthesia
- Slower the recovery from anaesthesia
MAC is the concentration of drug in the alveolar space which prevents a motor response to pain in 50% of patients. It’s inversely proportional to the potency. During general anaesthesia, a concentration of 1,2 – 1,3 x MAC is usually used. Inhaled anaesthetics have a depressive effect on the heart, respiration, and muscles.
Halothane, a halogenated hydrocarbon, is no longer used in the developed world due to the risk of halothane hepatitis, which has a very high mortality.
The halogenated ethers, isoflurane, desflurane, and sevoflurane, are the most widely used anaesthetic drugs. They vasodilate cerebral vessels, which increases the CBF and therefore the ICP, generally making them unsuitable for brain surgery. They all also have a cardiodepressive and bronchodilatory effects, the latter of which may be useful in an ICU setting. They also cause vasodilation in systemic vessels, causing hypotension.
Of these, isoflurane is most used. Isoflurane has the least cardiodepressive effect of the three.
Sevoflurane is often used for induction of anaesthesia, especially in children and in short interventions. All of them cause airway irritation, except for sevoflurane, so only sevoflurane can be used for induction of anaesthesia.
Nitrous oxide (N2O) is the least potent inhaled anaesthetic. It’s used for smaller procedures where complete loss of consciousness is not necessary, like during labour or dental procedures. It may be self-administered by the patient. It’s necessary to give pure O2 for a few minutes after the operation to prevent diffusional hypoxia. Repeated N2O abuse can cause B12 deficiency.
Intravenous anaesthetics
Intravenous anaesthetics have very fast onset of action (20 seconds). The anaesthetic effect stops when the drug redistributes from the CNS to other tissues. The duration of action is 5 – 10 minutes. All intravenous anaesthetics activate GABA receptors, except ketamine, which inhibits NMDA receptors.
Total intravenous anaesthesia (TIVA) refers to when only IV agents are used for anaesthesia. Propofol is the most commonly anaesthetic used for this.
Propofol is perhaps the most widely used IV anaesthetic. It’s the standard drug for induction of anaesthesia, it can be used for TIVA, or for sedation of patients in the ICU.
Etomidate has the least effect on the cardiovascular system, so it’s the preferred anaesthetic for patients with haemodynamic instability or heart conditions.
Ketamine is an NMDA receptor antagonist which induces a state of dissociative anaesthesia. It has a stimulatory effect on the cardiovascular system. It’s especially used in emergency situations with polytrauma, shock, severe asthma.
Benzodiazepines like midazolam, diazepam, lorazepam are used for conscious sedation during procedures like colonoscopies. They can be combined with opioids for general anaesthesia.
Barbiturates like thiopental and methohexital are used for patients who have increased intracranial pressure.
5. General anaesthesia and complications
General anaesthesia
General anaesthesia (GA) is the process of using drugs that act on the CNS to induce the 5 elements of anaesthesia, in order to facilitate invasive procedures. These 5 elements are:
- Loss of consciousness
- Absence of pain (analgesia)
- Blocking of noxious autonomous reflexes
- Loss of memory (amnesia)
- Relaxation of skeletal muscles
There are six stages:
- Preparation
- Induction – the transfer from wakefulness to narcosis
- Analgesic first (opioid), then anaesthetic (propofol)
- Rapid sequence induction (RSI) – for full stomach patients
- Delayed sequence induction (DSI) – for fasting patients
- Muscle relaxation
- In RSI – fast acting relaxants, like succinylcholine or rocuronium
- In DSI – slow acting relaxants, like atracurium, mivacurium
- Airway management
- Face mask, laryngeal mask/i-gel, or intubation
- Ventilation, monitoring and maintenance of general anaesthesia
- Reversal and recovery
- Reversal includes giving antidotes to the muscle relaxant
Complications during general anaesthesia
Three major factors, the lethal triad of death, is known for patients who’ve experienced major trauma, and they must be monitored for and treated by the anaesthesiologist. It includes hypothermia, acidosis, and coagulopathy. Each of these factors worsen the others in a positive feedback loop, and if all three appear the intraoperative mortality is 75%.
Treatment of the acidosis includes increasing the O2 delivery, giving blood in case of haemorrhage, and giving inotropes and vasopressors to increase the circulation.
Treatment of the coagulopathy includes giving tranexamic acid to prevent hyperfibrinolysis.
Treatment of hypothermia includes administering warmed saline infusions and applying blankets.
Malignant hyperthermia may occur in those with mutated ryanodine receptor. Suddenly increasing end-tidal CO2 is a warning sign for it. The treatment is dantrolene.
Other complications:
- Hypoxia
- Increase oxygen delivery
- Look for underlying cause
- Hypercarbia
- Look for malignant hyperthermia
- Hypotension
- Give 20 mL/kg crystalloid or blood product if fluid deficit
- Otherwise, give vasopressor
- Hypertension
- May be due to pain
- Deeper anaesthesia might be required
- Urapidil or labetalol also
- Bradycardia – give atropine or glycopyrrolate
- Tachycardia
- May be due to pain
- Deeper anaesthesia might be required
- Hypoglycaemia
- Hyperglycaemia – due to contrainsular hormones due to pain
- Trauma to airways from intubation
6. Pharmacology of muscle relaxants
Peripheral muscle relaxants are used for intubation and for ventilation of patients. They can only be used in patients who are already anesthetised.
They must be used with care in people with neuromuscular diseases like myasthenia gravis. Their effect can be monitored intraoperatively by train-of-four (TOF).
Non-depolarising muscle relaxants
- Atracurium
- Cisatracurium
- Rocuronium
- Vecuronium
- Mivacurium
Non-depolarising muscle relaxants are competitive inhibitors of acetylcholine at the nicotinic acetylcholine receptor.
Neostigmine and atropine can be used as an antidote for all of these. Vecuronium and rocuronium have a specific antidote called sugammadex.
Rocuronium has a fast onset of action and can be used for rapid sequence intubation.
Depolarising muscle relaxants
- Suxamethonium/succinylcholine
Suxamethonium is a nicotinic receptor agonist, which causes the skeletal muscle to depolarise and to stay depolarised. It’s mostly used for rapid sequence induction because of its fast onset of action.
7. Peripheral and central regional anaesthetic techniques: pharmacology, indications, contraindications
Regional anaesthesia refers to using local anaesthetics to render a specific area of the body anesthetised. It is used for operative anaesthesia but also for post-operative analgesia. They don’t cause loss of consciousness, but they may be combined with anaesthetics to achieve this.
Many adult surgeries can be performed with regional anaesthesia alone, without general anaesthesia. Regional anaesthesia is becoming more and more popular, as they have various advantages of general anaesthesia:
- It’s better for elderly
- Cheaper
- There’s no need for airway management, avoiding potential complications
- Less opioids are needed
- Less post-operative nausea and vomiting (PONV)
- Reduced postoperative pain
- Few cardiovascular and pulmonary side effects
There are various types:
- Central regional anaesthesia (neuraxial anaesthesia) – anaesthesia of the spinal cord
- Epidural anaesthesia
- In cervical, thoracic, or lumbar region
- Epidural catheter can remain in place for analgaesia beyond the surgery itself
- Onset of anaesthesia takes 10-20 minutes
- Spinal (subarachnoid) anaesthesia – for surgery below the umbilicus
- Only in lumbar region
- More rapid onset than epidural (immediate)
- More rapid haemodynamic changes than epidural
- Combined spinal-epidural anaesthesia
- Combines the advantages of both
- Epidural anaesthesia
- Peripheral regional anaesthesia – anaesthesia of peripheral nerves
- Major (multiple nerves or a plexus)
- Cervical plexus block
- Brachial plexus block
- Interscalene block
- Supraclavicular block
- Infraclavicular block
- Axillary block
- Minor (single nerve)
- Sciatic nerve block
- Femoral nerve block
- Major (multiple nerves or a plexus)
- Topical anaesthesia
- Intravenous regional anaesthesia
Intravenous regional anaesthesia (IVRA) or Bier block involves isolating an exsanguinated limb from the circulation with a tourniquet and injecting the local anaesthetic intravenously. It’s simple, safe, and effective, and especially suitable for short procedures and outpatient surgery. Prilocaine and lidocaine are typically used.
Pharmacology
- Lidocaine
- Prilocaine
- Bupivacaine
- Tetracaine
- Ropivacaine
These drugs block sodium channels, suppressing action potential development and nerve transmission. They’re lipid soluble, but after penetrating into the cells they become ionised, causing them to be trapped and activated. They selectively block thinner nerve fibres, blocking pain fibres but preserving motor fibres.
Systemic toxicity of local anaesthetics can occur if a lot is used, but the volumes of drug needed can be drastically reduced if the injection is ultrasound-guided, reducing the risk for toxic side effects. Bupivacaine is especially cardiotoxic. Side effects include bradycardia, AV block, CNS depression, metallic taste, arrhythmias.
Indications
Regional anaesthesia is preferable in:
- Oncological surgery
- Orthopaedic/traumatological surgery
- C-section
- Lower limb revascularisation
- Obese patients
- Elderly patients (prevents post-operative cognitive dysfunction (POCD)
- Paediatric surgery (in addition to GA)
Contraindication of central blocks
- Infection at injection site
- Patient refusal
- Increased ICP
- Coagulopathy
- Severe hypovolaemia
8. Patient monitoring during anaesthesia: depth of anaesthesia, peripheral muscle relaxation, gas exchange, circulation
The most important monitor during surgery is the anaesthesiologist. It’s important to observe the patient and heed the surgeon’s warnings. The monitoring machines are also used.
Some parameters are monitored in all cases of anaesthesia:
- SpO2
- ECG
- Non-invasive blood pressure
- FiO2 (the percentage of oxygen in the provided air)
- EtCO2 (end-tidal CO2)
Other parameters which are optional and can be monitored if necessary:
- Invasive blood pressure
- Temperature
- PaO2
- Diuresis monitoring
- Central venous cannulation
- Nasogastric tube
Depth of anaesthesia
Depth of anaesthesia can be measured by single-channel EEG-based systems, like the BIS (bispectral index). However, simple measurements like heart rate and blood pressure can give some indication as to whether the anaesthesia is too light and the patient is experiencing pain.
Peripheral muscle relaxants
The effect of peripheral muscle relaxants is evaluated by train-of-four (TOF). The equipment monitors the activity of the adductor pollicis muscle in response to four stimuli of the ulnar nerve.
Note that all sources I can find list the ulnar nerve as the innervator of this muscle, but according to at least three commenters, the examiners are adamant that it’s innervated by the radial nerve. The ABductor pollicis longus is innervated by the radial, but that is not the muscle measured here. All sources say that TOF monitoring involves the adductor pollicis and stimulation of the ulnar nerve. See here and here, for example.
Gas exchange
The EtCO2 is the concentration of CO2 at the end of expiration. It is evaluated by waveform capnography and is another indicator of the ventilation status. If it increases, the patient is hypoventilated.
The ratio between FiO2 and PaO2 is another good indication of the oxygenation status of the patient but requires ABG.
Circulation
The fluid intake (infusions) is monitored and compared to the fluid loss, usually as blood which is suctioned and as urine in the catheter, if present. During long-lasting open surgeries, insensible fluid loss must be taken into account as well.
9. Analgesics and their postoperative use
Management of post-operative pain is a major part of anaesthesiology and surgery. Post-operative pain not only is annoying to the patient, but it increases the risk of chronic post-surgical pain, AMI, arrhythmias, and poor outcomes in general. Post-operative pain occurs from the surgery itself, from the immobility and positioning during surgery, and from direct or indirect trauma to nerves.
Pain is not only the noxious stimulus, but also includes emotional, psychological, and social components. Fear or anxiety, for example, worsens pain.
Central sensitization to pain occurs during surgery and can worsen the postoperative pain or cause chronic pain. Preventing central sensitization is important in reducing pain.
Evaluation
It’s important to evaluate the patient’s postoperative pain, as all patients are different and experience pain differently. This can be done with a variety of different scales, especially the visual analogue scale (VAS).
Management
The WHO has a pain management ladder, which can be used in postoperative pain. The first step includes non-opioid analgesics, the second weak opioids, and the third and final step strong opioids. Adjuvants can be added at any step. However, it’s important to note that in very strong pain we can start at step 3, there’s no reason to try the other steps first.
Nowadays we prefer preventive analgesia, which is a multimodal approach which aims to prevent central sensitization by blocking all pain signals associated with the surgery, from the time of the incision to the final wound healing.
Regional anaesthesia is widely used to prevent postoperative pain. A catheter can be placed so that local anaesthetics can be applied for days or weeks. Nerve blocks can be placed at the end of the surgery to provide post-operative analgesia.
Nonopioids, including NSAIDs, COX-2 inhibitors, and paracetamol, are essential in reducing the need for opioids in postoperative pain.
Opioids have a variety of problems, but their use is often unavoidable with regards to postoperative pain. There are a variety of opioids available:
- Weak opioids
- Tramadol
- Dihydrocodeine
- Codeine
- Strong opioids
- Morphine
- Oxycodone
- Fentanyl (oral, IV, or transdermal patch)
Many adjuvant analgesics are effective in preventing and treating postoperative pain, like gabapentin, pregabalin, amitriptyline, etc. Gabapentin and pregabalin are especially useful for neuropathic pain.
Patient-controlled analgesia (PCA) refers to an infusion pump where the patient themselves can choose when analgesic is released. This allows the patient to spend less time in pain, and patients tend to use less analgesics than if following a set schedule. It is commonly used for postoperative and cancer pain.
Non-pharmacological modalities are also important, like psychotherapy, transcutaneous electrical nerve stimulation (TENS), physiotherapy, acupuncture, yoga, etc.
10. Chronic pain treatment
Chronic pain is the pain present for a longer period of time (> 3 months), and often continues after the cessation of the triggering factor or without a definite cause. It is a multidimensional issue and a disease in its own right. It’s a functionless pain, as it only reduces quality of life (whereas acute pain saves us from injury and teaches us).
Biological, psychological and social aspects of pain all influence perception of pain, and in turn are influenced by pain. Patients typically present with several issues besides pain, like exhaustion, depression issues, stress/anxiety, sleep issues, weak muscles, inactivity, loss of personal and social functioning, overuse of medicine, relationship issues, etc.
It’s a common problem, as it affects 10 – 30% of the EU.
Evaluation
Extensive pain interview, pain scales. It’s important to evaluate:
- The severity
- The impact on the patient
- The quality
- The time period
- Aggravating or alleviating factors
Treatment
The treatment is always multimodal, chronic pain cannot be treated only with medications. Options are described in the previous topic.
Hi! I got the topic “22. Definition and Ethical Aspects of Brain-Stem Death.” The head wanted to know how long we have to wait to confirm brain death. For primary, it was 7 hours (I think), and for secondary, it was 3 days.
Thank you so much for your notes!!!
Thank you for contributing! Added now.
Hello, thank you so much for the notes. Just wanted to say that in topic of Paediatrics Anaesthesia, I was asked how long before the surgery do you have to stop milk consumption. The answer was 3 hours.
Also, in the exam I said that you can give midazolam and dexmedetomidine as premedication, but the examiner said we almost never use dexmedetomodine, even though this was also mentioned in the booklet.
Thank you for your contributions!
For the critical care for poly trauma patients topic, could you please list the “Indications for damage control” list on your notes ? I failed the exam because I didn’t know that on that topic
Sorry that happened to you.
I watched the recording of our lecture on polytrauma, and “damage control surgery” was mentioned only in passing. Hussain, Lee, and Anne also just briefly mention it in their notes, so I’m not clear on what they wanted you to say.
I wrote a short paragraph with some general information about it. If you have any additional input, please share.
Thanks a lot, now that I finally passed the exam I can give some input, I’m sorry to say the exam has increased a lot in difficulty since the time you had it. The head of the department is an asshole who asks random questions form the booklet which no one studies. Even though most people including me still presented the topics word for word from your notes it’s still not good enough for him, and it’s pure luck/random that people pass 🙁 even tho you’re notes are great for a 5, for the head it’s not even enough for a 2 aparrently #poteproblems
Thank you for the comment. I didn’t even know there was a booklet.
Hello, regarding the damage control, i think the examiner specifically wants to hear these ones, which are in the booklet:
The care of bleeding patients has changed significantly in recent years with damage control resuscitation. Its core are:
1. Control the source of bleeding as early as possible
2. Treatment of coagulopathy
3. Acidosis prevention/correction
4. Hypothermia prevention/treatment
5. Cause minimal iatrogenia (dilution)
Also, the examiner also asks about operative and stabilising phases which are on the lecture slides.
Thanks again for your contributions! Added them now.
Epidural (spinal) anaesthesia
In cervical, thoracic, or lumbar region
Subarachnoid (spinal) anaesthesia – for surgery below the umbilicus
^ subarachnoid is spinal. epidural isn’t spinal.
Correct you are. fixed
Hi!
I had topic 8B today as well. The course director asked «where we all get the ulnar nerve from» and was very satisfied with the answer «deep radial nerve». I started with saying that different sources say different things (as i found one source saying ulnar nerve), he said that the only right answer is radial or specifically deep radial n.
The ABductor pollicis longus is innervated by the deep branch of the radial nerve, while the ADductor pollicis is innervated by the deep branch of the ulnar nerve. All sources say the TOF monitoring monitors the ulnar nerve and the adductor pollicis. The examiner either has wrong knowledge or they’re mixing up the adductor and the abductor.
Agree. As a board certified practicing anesthesiologist the ADductor pollicis is innervated by the deep branch of the ulnar nerve not the radial nerve. Deep radial innervates the ABductor pollicis and is NOT typically measured on TOF.
“The equipment monitors the activity of the adductor pollicis longus muscle in response to four stimuli of the ulnar nerve.”
It is innervated by the radial nerve
It’s not. See my other comment.
I got topic 8B and about the train of four and the examiner told me that the adductor policis longus is not innervated by the ulnar nerves is by the radial
He’s wrong, you can look it up.
Although I think the muscle may be called just adductor pollicis, without the longus
Hey!
Little heads up, in topic 7 under respiratory alkalosis the compensation will cause HCO3 to drop
<3
Thank you
“TCTs in case of thrombocytopaenia”
What does TCT stand for here? Cant find anything that makes sense
I was under the impression that TCT was a commonly used abbreviation for thrombocytes, but I must’ve mixed it up with something. Fixed now.
Thanks bro