Table of Contents
Page created on October 25, 2018. Last updated on December 18, 2024 at 16:56
We’ve discussed ventilation and diffusion. Now we’re going to talk about oxygen transport.
Oxygen transport in blood
Only 1.5% of the oxygen in the blood is dissolved in the blood fluid itself. The other 98.5% is bound to haemoglobin. The partial pressure of oxygen in a vessel measures the total “amount” of oxygen inside the vessel, both dissolved and bound to haemoglobin.
When the pO2 is normal (around 100 mmHg) is practically every oxygen-binding site of haemoglobin occupied by an oxygen molecule, meaning that the oxygen saturation of haemoglobin is 100%. When pO2 decreases will the saturation decrease as well, however not linearly.
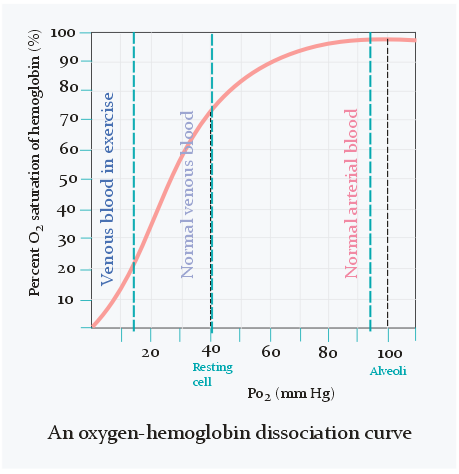
As seen on the figure above does the oxygen saturation change very little when pO2 is between 80 and 100 mmHg. It’s only when pO2 becomes lower than 80 that the saturation starts to decrease significantly and rapidly.
When oxygenated blood has reached an area that requires oxygen will the oxygen that is dissolved in the blood diffuse across the capillary wall to the interstitium. Oxygen in the interstitium is then taken up by the cells. Oxygen will then dissociate from haemoglobin to “refill” the dissolved oxygen in the blood that was just emptied.
In the veins is the oxygen saturation only 75%, which equals 40 mmHg of pO2, as seen on the figure above. Arterial blood contains 20 mL oxygen per 100 mL blood, while venous blood contains 15 mL O2 per 100 mL blood. The difference is only 5 mL. Oxygen content of blood can also be expressed as a volume percentage. For example, 20% of arterial blood is oxygen, while 15% of venous blood is oxygen. Because of this we can also write the difference between them as 5 percentage points.
This oxygen content difference is called ΔA-VO2, where the Δ means difference, A is arterial, V is venous and O2 is O2. As stated, it’s normally 5 mL O2 per 100 mL blood, but its value indicates the oxygen utilization of tissues and the value differs between different tissues in healthy people as well. In the coronaries the difference is 10 in rest and 14 in work, while in the kidney it is less than 2.
It’s not technically correct to say that the ΔA-VO2 is 5%, because that would imply that the difference in oxygen content is 5%, which it’s not, as a commenter pointed out. 15 is 75% of 20. It works if it’s 5 percentage points rather than 5 percent. To avoid confusion, it’s best to just use mL O2 per 100 mL blood instead.
CO2 is transported in a different way. Carbon dioxide diffuses from the interstitium to the plasma and then into the RBCs. Inside will carbonic acid (H2CO3) be formed, which diffuses back to the plasma. When the blood reaches the lung will the opposite process occur, and plasma CO2 will diffuse into the alveoli.
Here’s a table of important values to remember.
|
Parameters |
Outside air | Alveoli | Arterial blood | Tissues |
Mixed venous blood |
|
pO2 |
150 mmHg | 100 mmHg | 96 – 100 mmHg | 40 mmHg | 40 mmHg |
|
Hb content |
140 – 160 g/l |
140 – 160 g/l |
|||
|
Hb O2 saturation |
98% |
75% |
|||
|
O2 content |
20 mL / 100 mL blood |
15 mL / 100 mL blood |
|||
|
ΔA-VO2 |
5 mL / 100 mL blood |
||||
| pCO2 | ≈ 0 mmHg | 40 mmHg | 40 mmHg | 46 mmHg |
46 mmHg |
“Mixed venous blood” is the blood that normally enters the pulmonary trunk, meaning that it is the mix of all venous blood in the circulation before entering the heart.
Abnormalities of oxygen transport
Four major factors can modify the haemoglobin-oxygen dissociation curve. They’re best expressed on a figure.
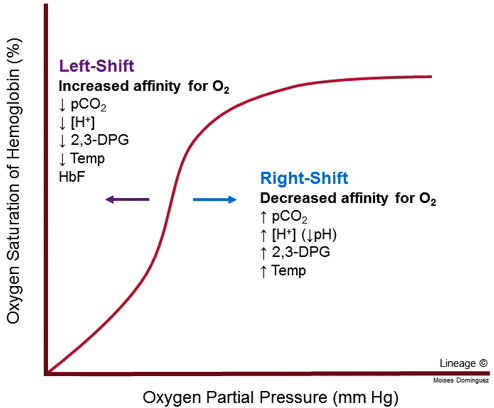
A nice rule to remember is that a decrease in any of the variables cause a left shift while an increase in any variable causes a right-shift. But what does left-shift and right-shift mean?
When the curve is left-shifted is the affinity of haemoglobin to oxygen increased, meaning that haemoglobin binds oxygen more easily and doesn’t let go of oxygen as easily. This is not a good thing, as this decreases the amount of oxygen released to the tissues in the periphery.
When a curve is right-shifted is the affinity to oxygen decreased. This causes oxygen to dissociate from haemoglobin more easily, which can be a good thing as more oxygen is released to the tissues in the periphery. Right-shift occurs physiologically during exercise, as muscles produce more CO2 and lactic acid, which shifts the curve to the right, which causes the muscles to receive more oxygen in return.
Some abnormal haemoglobin types can shift the curve to the right or left as well. They are:
| Shift to the left | Shift to the right |
| Hb Rainier | Hb Kansas |
| Hb Barts | Hb Seattle |
| Hb H | Hb S (in sickle cell disease) |
| Carboxyhaemoglobin | |
| Methaemoglobin | |
| Foetal Hb | |
| Diabetes mellitus |
Most are hereditary disorders however the ones in cursive are not. Foetal haemoglobin and diabetes mellitus decrease the binding of 2,3-BPG to haemoglobin, effectively causing a left-shift.
Carboxyhaemoglobin is a complex of Hb and carbon monoxide, formed in CO poisoning. Methaemoglobin is found in small amounts in every person. When its level increases for any reason will there be a shift to the left.
CO poisoning
Carbon monoxide is an odourless, colourless gas. It has a 200 times higher affinity to haemoglobin than oxygen does, forming the carboxyhaemoglobin complex. It’s therefore understandable that high amounts of CO aren’t needed to get poisoned.
Symptoms include nausea, headache, malaise and muscle weakness. The major consequence is severe hypoxia. Treatment is by administrating a mixture of 95% O2 and 5% CO2. The CO2 is used to maintain the respiratory drive. Death usually occurs when over 60% of all haemoglobin is carboxyhaemoglobin.
Methaemoglobin
As stated earlier, small amounts of methaemoglobin exist even in healthy people. This type of Hb contains a ferric (Fe3+) instead of a ferrous (Fe2+) ion. Ferric haemoglobin (met-Hb) does not bind oxygen. If one iron ion in haemoglobin becomes oxidised to ferric iron, the oxygen affinity of the remaining three (ferrous) iron ions will be increased. This simulates a left-shift in the haemoglobin oxygen dissociation curve. These two mechanisms together lead to tissues not receiving enough oxygen.
In healthy humans will the enzyme methaemoglobin reductase convert met-Hb to normal Hb. Some people have a deficiency in this enzyme, causing the met-Hb levels to rise and their skin to be blue.
The oxidation of Hb to met-Hb can be due to high amount of nitrates in the food, or oxidizing drugs. This is especially dangerous for new-borns, as their methaemoglobin reductase enzymes aren’t sufficient yet.
Hello abu greek
You mentioned “Met-hb binds oxygen too strongly”.
According to the internet “ When hemoglobin iron becomes oxidized to the ferric state (Fe3+), it is no longer able to bind oxygen and is called methemoglobin.“ – Sciencedirect.com, it explains the cyanosis.
Thank you!
Well spotted. Corrected now.
Hey
“Arterial blood contains 20 mL oxygen per 100 mL blood, while venous blood contains 15 mL O2 per 100 mL blood. The difference is only 5 mL, or 5%.”
The difference would be 25% not 5!
And btw Thank u very much! You are a lfe savior ❤️
Yeah, it should be 5 percentage points, not 5 percent. The book (p. 105) confused me. I’ll fix it.
I’m glad to have helped.