Table of Contents
Page created on December 17, 2018. Last updated on December 18, 2024 at 16:56
Basics
In arterial plasma is the concentration of H+ around 4 x 10-8 moles per litre. pH = -log([H+]), so the plasma pH is around -log(4 x 10-18) = 7.4 approximately. Indeed is the normal range of pH in the blood between 7.35 and 7.45, although pH down to 6.8 and up to 7.8 is survivable.
The intracellular pH in the cytoplasm is around 7.2, but the pH in other organelles differ. An important thing to remember about pH balance is that the goal isn’t to keep the pH of the plasma within normal range but rather to keep the pH of the cells normal. It turns out however that, because all fluid compartments of the body are interconnected, does a normal plasma pH mean a normal cell pH in most cases.
Bicarbonate = [HCO3–]
Carbonic acid = [H2CO3]
Buffers
The body uses acid-base buffers to keep the pH within normal levels. The body has multiple buffers:
- Carbonic acid / bicarbonate buffer – HCO3– / H2CO3
- Phosphate buffer – HPO42- / H2PO4–
- Plasma proteins
- Haemoglobin
- Intracellular buffers
The carbonic acid buffer is by far the most important, however also a bit complicated, because it exists in an equilibrium, like this:
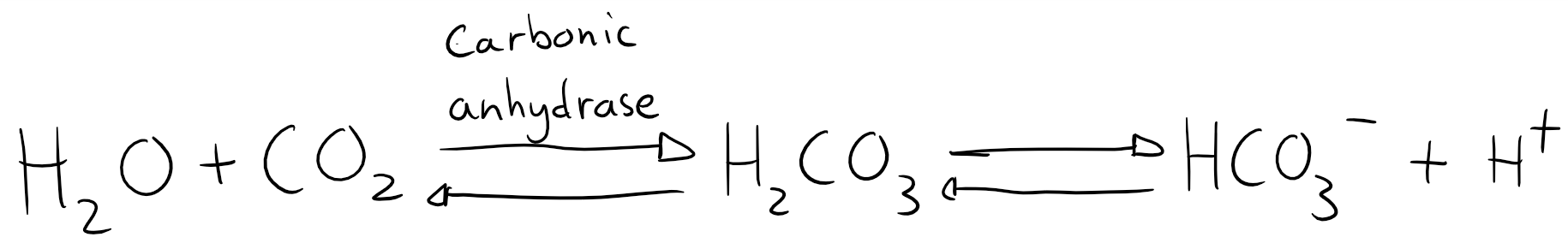
In plasma is the level of H2CO3 very low. Any H2CO3 is either dissolved into HCO3– or split into H2O and CO2 by carbonic anhydrase. The equation can therefore be simplified to look like this:
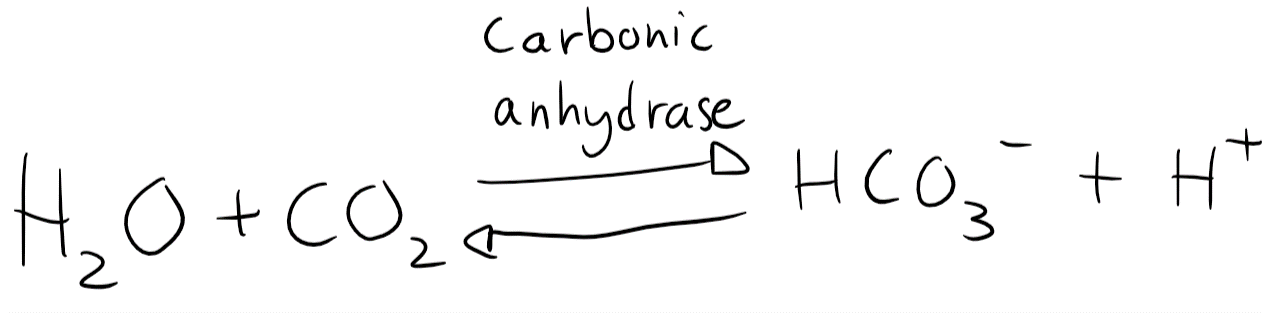
You probably remember the Henderson-Hasselbalch equation. It looks like this:
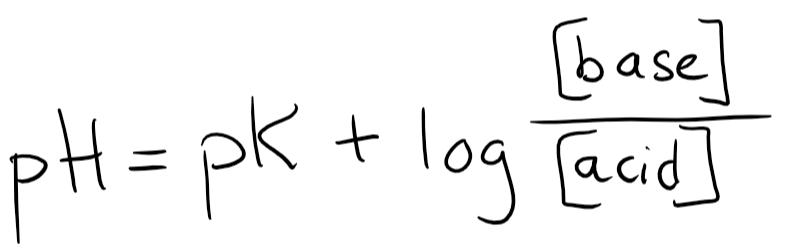
Let’s plot in the pK for H2CO3, which is 6.1. Let’s also add the base (HCO3–) and the acid. However, since H2CO3 is converted into CO2 by carbonic anhydrase will we write CO2 as our acid instead of H2CO3.
But CO2 is a gas, not a solute. The only way to measure the level of CO2 in the blood is by partial pressure. To input the partial pressure into this equation we need to convert partial pressure into a unit of concentration. The explanation isn’t important, but to use CO2 in this equation we must multiply the pCO2 by 0.03. The equation now looks like this:
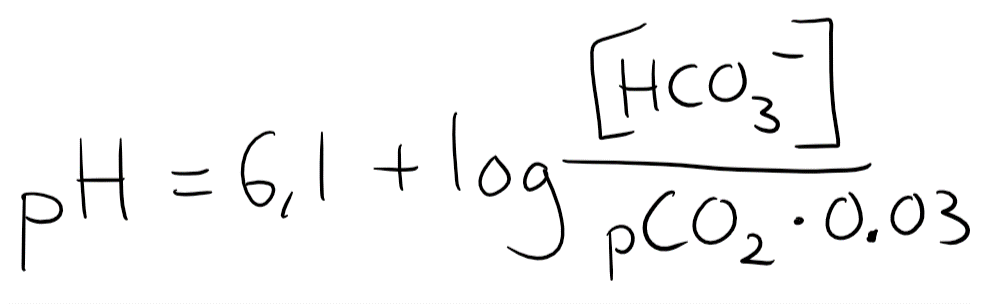
The normal value of [HCO3–] is 24 mM, while the normal pCO2 is 40. 40 x 0.03 = 1.2, so we’ll input that.
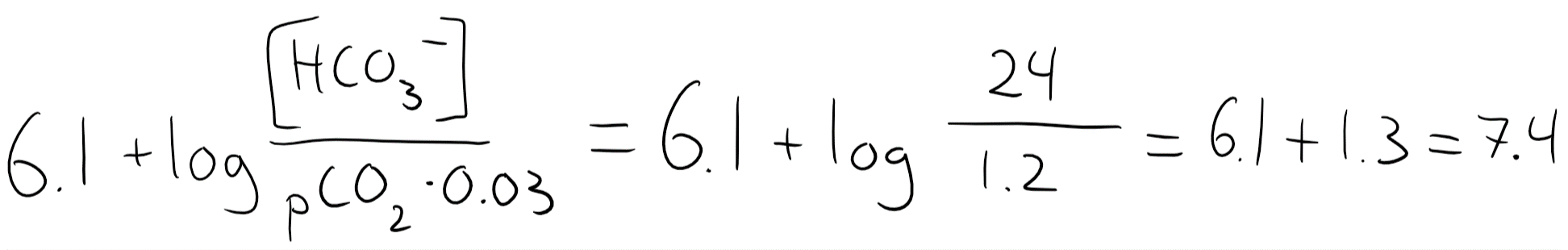
So when [HCO3–] and pCO2 are both normal is the pH 7.4. You should remember that 24:1.2 = 20:1
Another important thing to notice here is that the level of pCO2 and [HCO3–] isn’t directly responsible for the pH, but rather the ratio between the two, 20:1. In fact, in many pathological cases will one of these parameters increase or decrease, which ruins the 20:1 ratio. The body will compensate for this by increasing or decreasing the other parameter accordingly, to try to maintain the 20:1 ratio and therefore the normal pH.
Let’s take an example. You start hyperventilating. This decreases the pCO2, which makes the [HCO3–]:[pCO2] ratio higher than 20:1. This means that the pH increases. What can the body do to compensate? The kidney will excrete more HCO3–. This returns the ratio toward the normal 20:1, which causes the pH to get closer to normal.
This buffer is a very good one, as the body can regulate both pCO2 and [HCO3–] by two separate mechanisms, respiration and excretion respectively. There are also large amounts of both in the blood, so the buffer has a large buffer capacity.
The other buffers aren’t as important. They mostly work when the carbonic acid level is acutely increased or decreased. The bones however are actually important parts of the buffer, as they store a lot of phosphate, which comprises the phosphate buffer.
The plasma buffers works almost immediately after the pH change. The intracellular buffer however may take 6 – 8 hours to kick in.
Important terms
There are some important terms in connection with this we should know.
There are two ways to express the level of bicarbonate in the blood. We can express the actual bicarbonate, which is the actual number of bicarbonate molecule in the blood. The other way is standard bicarbonate, which what the number of bicarbonate molecules would have been if the pCO2 level was normal.
Standard bicarbonate is measured by putting the serum sample in a machine that changes the pCO2 level of the sample to 40 mmHg. It then measures the bicarbonate concentration. This is therefore a measurement that is “standardized” to 40 mmHg pCO2, hence standard bicarbonate.
It’s important to distinguish the two because bicarbonate and pCO2 depend highly on each other, as they’re in an equilibrium. If you slow down your breathing will pCO2 increase. Because CO2 and bicarbonate are in an equilibrium will some of the extra CO2 molecules be converted into bicarbonate so that the actual bicarbonate increases. However, nothing has actually happened that increases bicarbonate by itself – it was just raised because pCO2 was. We need a method to measure what the bicarbonate would be if the pCO2 level is normal. This is where the standard bicarbonate comes in. The standard bicarbonate in this case would be unchanged.
Standard bicarbonate allows us to measure whether the bicarbonate increase (or decrease) is due to a primary change in pCO2 (a respiratory condition. Standard bicarbonate would be unchanged) or due to a primary change in bicarbonate (a metabolic condition. Standard bicarbonate would be abnormal).
An example where standard bicarbonate would increase would be if there was a reduction in bicarbonate excretion in the kidneys for example.
Buffer base (BB) is a measurement of the total amount of buffer in the plasma. It’s the sum of standard bicarbonate, haemoglobin and other buffers in the blood, and the normal value is 46 mmol/L.
The base excess (BE) measures how much the buffer base has deviated from the normal value of 46 – 48 mmol/L. For example, if the buffer base is 45 mmol/L would the base excess be -1. If the buffer base is 48 would the base excess be 2.
Two organs are essential in pH homeostasis: the lungs and the kidneys.
Regulation of pH by the lungs
The lungs are important in the regulation. At rest is 20 000 mmol CO2 eliminated by respiration daily. To give you an example of how important the lungs are: If respiration was stopped for 20 minutes would pH fall below 7.0.
Increased metabolism, such as during exercise, produces a lot of CO2. However, healthy lungs can easily excrete more CO2 than the metabolism can produce. By increasing respiratory frequency and depth can the lungs increase the CO2 elimination to accommodate the increased CO2 production.
The lungs are good at compensating for metabolic acidosis, as they can simply increase the ventilation. However, they’re not as good at compensating for metabolic alkalosis. In theory can they decrease ventilation to withhold more CO2, but that decreases pO2 as well. When the pO2 level reaches 60 mmHg would the hypoxaemia start to increase the respiratory drive, limiting further compensation.
Ventilatory compensation can start relatively quickly after the pH change. It reaches maximum capacity around 24 hours after the change. Renal compensation starts later, as we will see.
Regulation of pH by the kidneys
The kidneys don’t excrete as much acid as the lungs, just more than 35 mmol H+ equivalent daily, potentially up to 100 mmol if needed. The kidneys can normally make the urine pH as low as 4.4, but not lower.
The kidney has three mechanisms to regulate pH. The net result of them is that H+ is excreted and that sodium bicarbonate is reabsorbed.
You should know that the details of the three mechanisms aren’t really important. You shouldn’t learn the drawings and ion transports by heart, but you should know what goes on in each mechanism and which RTA corresponds to which mechanism.
Mechanism 1 occurs in the proximal tubular cells and mostly does bicarbonate reabsorption. It works like this:
- The tubular cell uses carbonic anhydrase to convert intracellular CO2 + H2O -> HCO3– + H+
- H+ is excreted into the tubular lumen in exchange for Na+
- Intracellular HCO3– and Na+ is absorbed through the basolateral membrane into the capillaries (This step doesn’t work in RTA II)
- Inside the filtrate is H+ combined with HCO3– to form CO2 and H2O
- CO2 is a gas and therefore diffuses back into the tubular cell and further into the capillaries
- CO2 is eliminated by respiration
| Substance | Molecules after step 2 | Molecules after step 5 |
| Na+ | 1 in the filtrate | 1 in the blood |
| HCO3– | 1 in the filtrate, 1 in the cell | 1 in the blood |
| CO2 | 0 | 1 in the blood (converted from HCO3–) |
| H+ | 1 | 0 |
| H2O | 0 | 1 in the filtrate |
The net result is that 1 HCO3– and 1 Na+ has been absorbed from the filtrate into the blood.
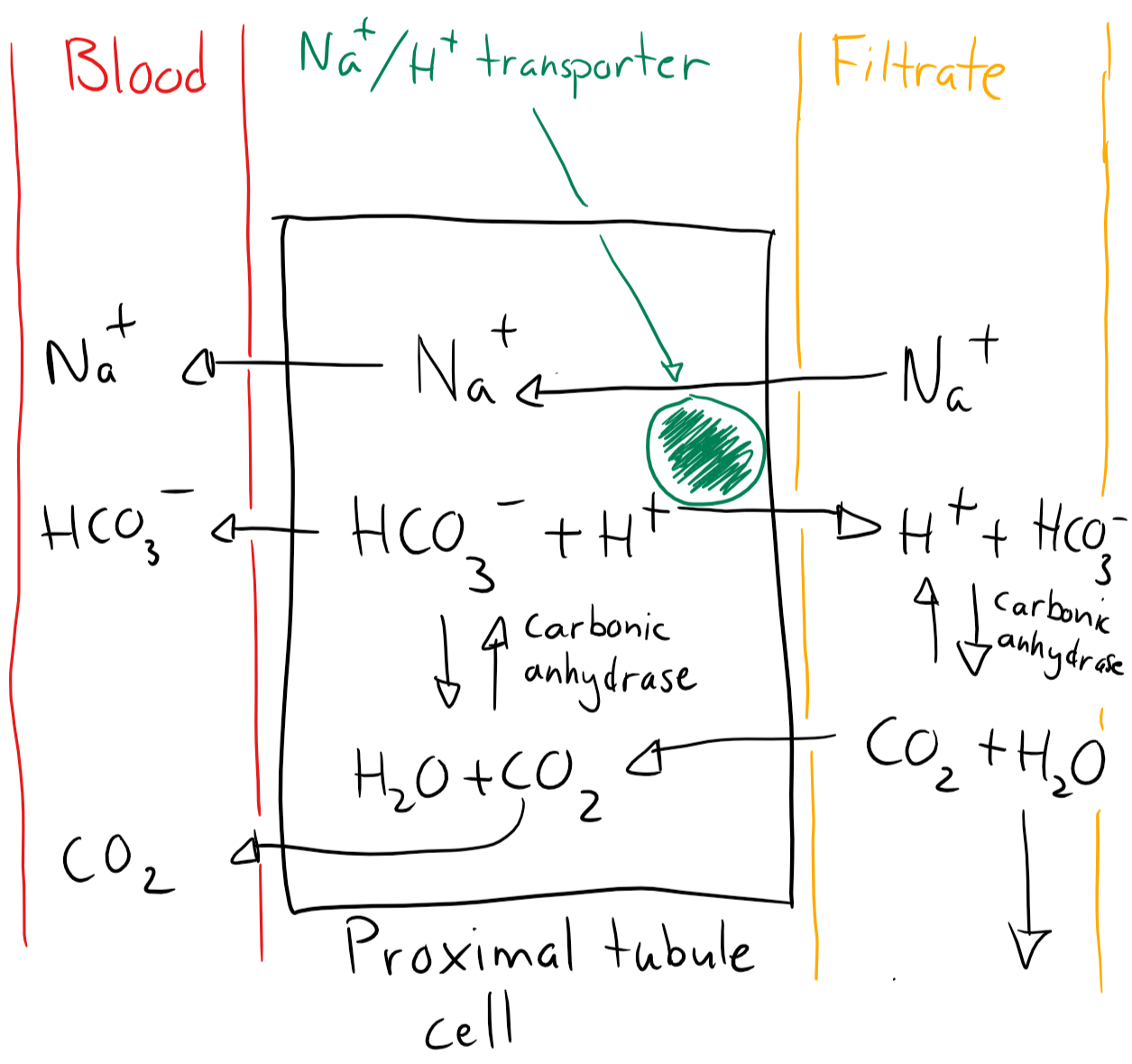
Mechanism 1. Bicarbonate reabsorption in proximal tubule
Mechanism 2 occurs in the distal tubules and mostly excretes H+. It works like this:
- The cell uses carbonic anhydrase to produce HCO3– and H+ from CO2
- The cell excretes H+ by active transport (This step doesn’t work in RTA I)
- The cell absorbed Na+ to maintain electroneutrality
- Intracellular Na+ and HCO3– is absorbed into the capillaries
| Substance | Molecules after step 2 | Molecules after step 4 |
| Na+ | 1 in the filtrate | 1 in the blood |
| HCO3– | 1 in the cell | 1 in the blood |
| CO2 | 0 | 0 |
| H+ | 1 in the cell | 1 in the filtrate |
The net result is that 1 CO2 has been converted into HCO3– and H+. The former has entered the blood while the latter is excreted in filtrate. Na+ is also reabsorbed from the filtrate.
Note that the HCO3– wasn’t reabsorbed from the filtrate – it was generated inside the cell. This helps to improve the plasma bicarbonate level.
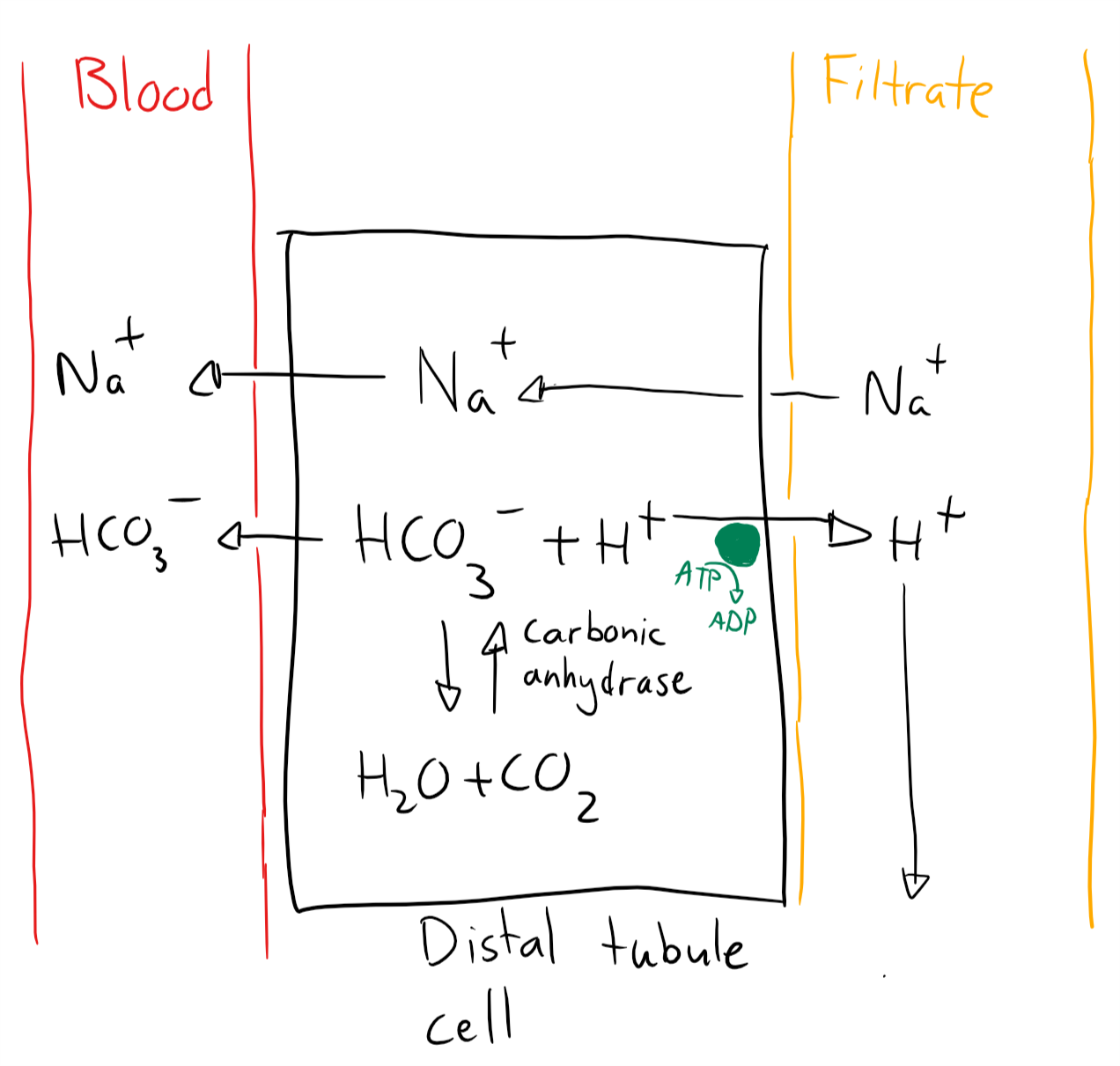
Mechanism 2. Proton excretion in the distal tubule
Mechanism 3 also occurs in the distal tubules and excretes ammonia that binds to H+ in the filtrate to make the filtrate less acidic, to prevent it from damaging the tubules.
- NH3 is produced from glutamine inside tubular cells
- NH3 is excreted into the filtrate
- NH3 combines with H+ in the filtrate to form NH4+
The result is that there are fewer H+ in the filtrate, so it’s not as acidic. As a bonus did the kidney get rid of some toxic NH3 too!
The net result of all three mechanisms is that H+ is excreted and sodium and bicarbonate is reabsorbed. These processes are activated by high pCO2 (respiratory acidosis), or a decrease in bicarbonate (metabolic acidosis). They’re inhibited by alkalosis.
This kidney compensation is a slow process. It needs 24 hours to start, and 72 hours to reach maximal capacity.
Hi Nikolas,
“An example where standard bicarbonate would increase would be if there was a reduction in bicarbonate excretion in the kidneys for example.”
Wouldn’t the standard bicarbonate decrease, if the excretion in the kidneys were reduced?
If the kidneys excrete less bicarbonate, i.e. they remove less bicarbonate from the body, wouldn’t you agree that the bicarbonate level in the body would increase?
Silly me must have worked to long hours yesterday. I do NOT know what I was thinking!
As always, I’m grateful!
No worries! Happens to me too.
Can someone tell me why the hell they called it puffer? Just a typo they never got around to fix? lol
Hahah, I never noticed that. Just like the 100 other mistakes they didn’t care to fix.
In Hungarian we use “puffer” instead of “buffer”, maybe that’s the origin of the mistake.
Hey dude
the normal value of buffer base in the MRTs is 48mmol/L 😅
The normal value is 46 – 48, so yeah. Fixed
Hello !! firstly thanks for everything !! your notes are more than amazing !! and you make our life easier !!
and are you sure we don’t really need to know the rest of the details ?
Thanks !! 🙂 🙂
Thank you so much for the kind words! Words like yours make it worthwhile to write notes.
I take back what I wrote when it said previously. I’ve rewritten that statement. It never hurts to know details but it’s most important to learn the most important stuff first, and the details maybe later. However, I’m sure you could get a 5 at that topic without being able to draw that figure.
Hi, Nik!
During the seminars, our teacher emphasized that it was very likely to get question about how long time it takes for the different buffers to work.
EC: <1h
IC: 6-8h
Respiration: 12h
Kidney: 24-72h
Done. Thanks!