Table of Contents
Page created on March 7, 2018. Last updated on December 18, 2024 at 16:56
I’ve given up on making summaries by now. Here’s a table of some of bloods properties.
| Volume | 5 – 6L for males, 4 – 5L for females |
| Viscosity | 4 – 5 times thicker than water |
| pH | 7.35-7.45 |
| Osmolarity | 300 mOsm |
| Salinity | 0.85% |
| Temperature | 38°C |
There are multiple families of proteins in the blood. They are, in order from most to least abundant: albumins, globulins, fibrinogen, regulatory proteins, and lastly, clotting factors.
The globulins can be divided into four groups: the α1-globulins, the α2-globulins, the β-globulins and the γ-globulins. The alpha and beta globulins are mainly (but not exclusively) used for transport purposes while the gamma globulins are the immunoglobulins (IgG, IgE and so on).
Albumin has three functions. To create oncotic pressure, to work as a buffer and to transport insoluble molecules, like thyroid hormones, retinol, calcium, drugs, free fatty acids, bilirubin, and so on.
A protein called prealbumin is a transporter for thyroid hormones and retinol.
Acute phase proteins
Certain stresses, like infection, inflammation, trauma or surgery, causes the body to produce certain cytokines (IL-1, IL-6 and TNF-α) that causes the liver to increase production of some plasma proteins. These are called positive acute phase proteins and are α1-antitrypsin, haptoglobin, ceruloplasmin, fibrinogen, and C-reactive protein.
α1-globulins
The α1-globulins are prothrombin, α1-antitrypsin and α1-fetoprotein.
Prothrombin is a glycoprotein which is essential for blood-clotting.
α1-antitrypsin is a protein found in blood that inhibits the function of proteases (enzymes that degrade proteins). It’s used to protect the tissues from the damage caused by neutrophil elastase, an enzyme secreted by neutrophils to break down bacteria. Because proteases cannot differentiate between bacterial proteins and host proteins, we have α1-antitrypsin in our blood to turn them off after they’ve broken down the bacteria.
The last α1-globulin is α1-fetoprotein, or AFP. AFP is a glycoprotein that is basically the fetal form of albumin. When found in considerable levels in (non-pregnant) adults, it can be indicative of a tumor.
α2-globulins
The α2-globulins are ceruloplasmin, haptoglobin and α2-macroglobulin.
Ceruloplasmin carries copper in the serum. It also inactivates reactive oxygen species.
Haptoglobin binds free hemoglobin to avoid it being filtered into the urine, which would be a waste of iron, and to inhibit free hemoglobin’s oxidative activity. The haptoglobin-hemoglobin complex is broken down and recycled in the spleen.
α2-macroglobulin is a protease inactivator. It also acts as a carrier protein for several proteins, like insulin, basic fibroblast growth factor and TGF-β.
β-globulins
The β-globulins are C-reactive protein, transferrin and β2-microglobulin.
C-reactive protein, or CRP, is a protein that binds to phosphocholine which is found on the cell membrane of dead cells and some bacteria. This binding activates the complement system. CRP also enhances phagocytosis of whatever CRP binds to. CRP is therefore an opsonin. It’s elevated whenever there is inflammation.
Transferrin is the transporter of iron in blood. You can read more about it in topic 9.
β2-microglobulin is a component of the MHC I molecule.
Serum protein electrophoresis
The levels of the different proteins found in the serum can be determined by electrophoresis. A serum protein electrophoresis of a healthy person results in a gel (on the bottom, blue) and graph (on the top) that looks like this:
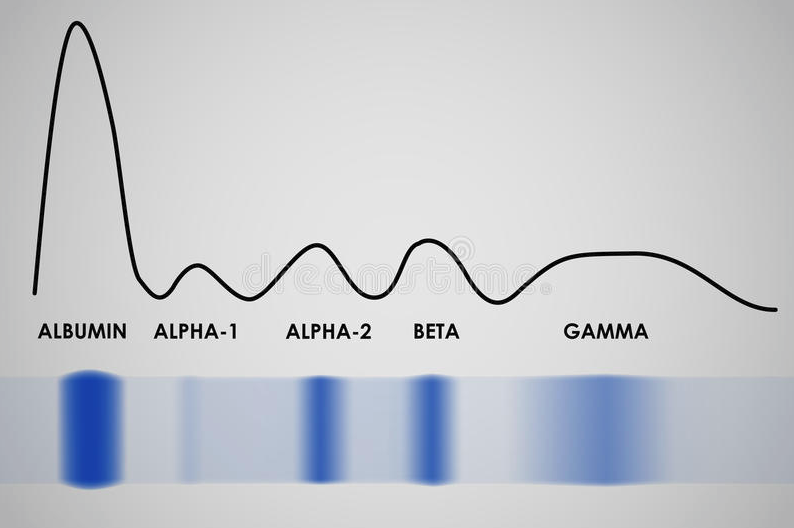
The different proteins travel along the gel and settle in five different bands (the stripes of blue). The graph is made based on the gel. Where the color of the gel is most intensive the amount of protein is higher and therefore the graph is higher. This is most easily seen on the first peak on the graph: the protein that is most abundant in the serum is albumin, so it gets the most intense (most blue) band on the bottom and therefore the highest peak on the graph.
As you can see on the gel, there are five bands that each correspond to one peak on the graph and there are five peaks. Each of these peaks represents a group of protein found in the serum and the height of the peak shows the amount of the proteins in that group that can be found in the serum.
The table below shows which serum proteins are found in which bands.
| Band | Plasma proteins |
| Albumin | Albumin |
| Alpha-1 | α1-antitrypsin
Prothrombin α1-Fetoprotein |
| Alpha-2 | Ceruloplasmin
Haptoglobin α2-macroglobulin |
| Beta | C-reactive protein (CRP)
Transferrin β2-microglobulin |
| Gamma | Immunoglobulins (antibodies)
IgA, IgE, IgG, IgM |
Because CRP is elevated when there is inflammation, the serum protein electrophoresis of a person who is ill would show a higher “beta” peak than for a healthy person. In the case where the body produces a lot of antibodies will the “gamma” peak be much higher than normal.