Table of Contents
Page created on March 14, 2018. Last updated on December 18, 2024 at 16:55
Learning objectives
- What are nucleotides?
- Which pyrimidine bases exist?
- Which purine bases exist?
- Which molecules are necessary for de novo synthesis of purines?
- What are the steps of de novo synthesis of purines?
- Which molecules are necessary for de novo synthesis of pyrimidines?
- What are the steps of de novo synthesis of pyrimidines?
- What are the differences between CPS1 and CPS2?
- Describe the salvage pathway.
- Describe the degeneration of purines
- What is the pathomechanism of gout?
- What drug is used to treat gout, and how does it work?
Nucleotides
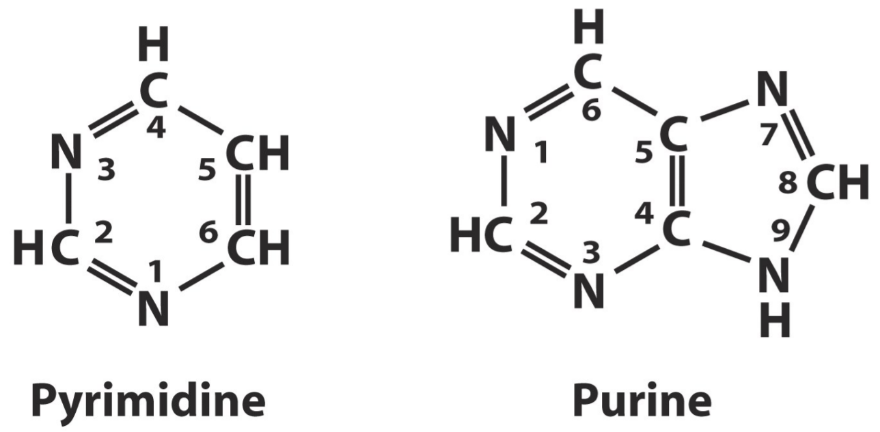
The two classes of bases that can comprise a nucleotide. Note that purines are just pyrimidines with an extra ring.
Nucleotides are biomolecules consisting of a base, a pentose, and a phosphate group. The same biomolecule without the phosphate group is called a nucleoside.
There are two types of bases; pyrimidine bases and purine bases.
Pyrimidine bases consist of one ring. There are three pyrimidine bases, cytosine (C), thymine (T), and uracil (U).
Purine bases consist of two rings. There are four purine bases, adenine (A), guanine (G), hypoxanthine, and xanthine.
RNA (ribonucleic acid) consists of C, U, A, and G nucleotides. DNA (deoxyribonucleic acid) consists of C, T, A, and G deoxyribonucleotides, referring to the fact that the ribose sugar of these nucleotides has been deoxidised (reduced), forming deoxyribose.
ATP is comprised of adenine and three phosphates. GTP is comprised of guanine and three phosphates.
Hypoxanthine is the base of IMP, and xanthine is the base of XMP. IMP and XMP are intermediates of de novo biosynthesis of purines, and metabolites of purines.
Nucleotides can be synthesised de novo (“from the beginning”), or they can be salvaged (“recycled”) from biomolecules which contain them, like DNA and RNA.
Nucleotide synthesis is a limiting factor of DNA synthesis and therefore cell proliferation. Many anticancer drugs and antibiotics inhibit nucleotide synthesis, which indirectly inhibits cell proliferation.
De novo biosynthesis of purines
During de novo synthesis of purines the basic ring structure is synthesised while bound to the ribose phosphate.
Aspartate, 2 formate, 2 glutamines, glycine, and CO2 are required for the de novo synthesis of purines.
Step 1: synthesis of PRPP
De novo synthesis begins with the synthesis of PRPP from ribose 5-phosphate by PRPP synthetase. This requires the conversion of ATP to AMP, which equals 2 ATP equivalents. PRPP is the source of the ribose sugar of the nucleotide.
Step 2: synthesis of IMP
After 10 more steps, PRPP is converted into inosinate (IMP). These steps require 2 formate, 1 glycine, 1 CO2, 1 aspartate, 2 glutamine, and 5 ATP.
Step 3: synthesis of AMP or GMP from IMP
The synthesis of AMP from IMP occurs in two steps, while the synthesis of GMP from IMP occurs in two different steps. Synthesis of AMP requires GTP and aspartate. Synthesis of GTP requires ATP and glutamine.
Regulation
De novo synthesis of purines requires a lot of energy and is therefore tightly regulated so that it’s kept to the minimum level needed. Regulation is by negative feedback inhibition.
De novo biosynthesis of pyrimidines
During de novo synthesis of pyrimidines the basic ring structure is synthesised first and then bound to the ribose phosphate. This is in contrast to de novo synthesis of purines.
Glutamine, aspartate and CO2 are required for pyrimidine synthesis.
Step 1: Synthesis of UMP
It begins with the synthesis of carbamoyl phosphate from glutamine and bicarbonate. This is the carbamoyl phosphate synthetase 2 (CPS2) reaction, which occurs in the cytosol.
Carbamoyl phosphate is attached to aspartate, forming carbamoylaspartate. A few more reactions yields the pyrimidine ring, and after attachment of ribose 5-phosphate from PRPP, UMP is finished.
CPS1 occurs in the mitochondria and belongs to the urea cycle.
Step 2: Synthesis of UTP and CTP
Kinases convert UMP to UDP to UTP. UTP can then be converted to CTP by amination.
Step 3: Synthesis of dUMP and dTMP
CDP is converted to dCDP (deoxy-CDP) by ribonucleotide reductase. A kinase converts dCDP to dCTP. A deaminase converts dCTP to dUTP. dUTPase converts dUTP to dUMP. Thymidylate synthase converts dUMP to dTMP.
The salvage pathways
In the salvage pathways, phosphoribosyltransferases react PRPP with free bases to yield a nucleotide, for example: adenine + PRPP -> AMP + PPi. The brain is especially dependant on salvage pathways.
If the salvage pathways are defect, such as in Lesch-Nyhan syndrome, the de novo pathways will be activated. Instead of being salvaged the bases will be metabolised into uric acid, causing hyperuricaemia. Lesch-Nyhan is a condition caused by deficiency of the enzyme which salvages hypoxanthine and guanine, hypoxanthine-guanine phosphoribosyltransferase.
Degradation
Degradation of purines
GMP is converted to guanosine, which is converted to guanine. Guanine is deaminated to xanthine.
AMP is converted to adenosine -> inosine -> hypoxanthine. Hypoxanthine is converted to xanthine by the very important enzyme xanthine oxidase.
Xanthine is then converted to uric acid by xanthine oxidase again. Other animals can further metabolise uric acid, but humans cannot. Uric acid is excreted by the urine.
Degradation of pyrimidines
Unlike purines, pyrimidines can be metabolised completely into ammonium and urea.
Gout
Uric acid is relatively insoluble. In abnormally high serum concentrations of uric acid it can precipitate as painful urate crystals, which causes the disease gout. These crystals precipitate in joints, most commonly the joints of the toes, causing pain and joint destruction.
The underlying cause may be genetic under-excretion of urate, but the most common cause is a diet which contains purine-rich foods and alcohol.
Gout is treated with allopurinol, a drug which inhibits xanthine oxidase. By inhibiting this enzyme hypoxanthine and xanthine are never converted to uric acid, and are instead salvaged or excreted directly.
Summary
- What are nucleotides?
- Nucleotides are biomolecules consisting of a base, a pentose, and a phosphate group.
- Which pyrimidine bases exist?
- Cytosine (C), thymine (T), and uracil (U)
- Which purine bases exist?
- Adenine (A), guanine (G), hypoxanthine, and xanthine
- Which molecules are necessary for de novo synthesis of purines?
- Aspartate, 2 formate, 2 glutamines, glycine, and CO2
- What are the steps of de novo synthesis of purines?
- Step 1: synthesis of PRPP
- Step 2: synthesis of IMP
- Step 3: synthesis of AMP or GMP from IMP
- Which molecules are necessary for de novo synthesis of pyrimidines?
- Glutamine, aspartate and CO2
- What are the steps of de novo synthesis of pyrimidines?
- Step 1: Synthesis of UMP
- Step 2: Synthesis of UTP and CTP
- Step 3: Synthesis of dUMP and dTMP
- What are the differences between CPS1 and CPS2?
- CPS1 is involved in the urea cycle, uses ammonium as substrate, and is located in mitochondria
- CPS2 is involved in de novo biosynthesis of pyrimidines, uses glutamine as substrate, and is located in cytosol
- Describe the salvage pathway
- Phosphoribosyltransferases react PRPP with free bases to yield a nucleotide
- Describe the degeneration of purines
- GMP -> guanosine -> guanine -> xanthine
- AMP -> adenosine -> inosine -> hypoxanthine
- Xanthine oxidase converts hypoxanthine to xanthine and xanthine to uric acid
- What is the pathomechanism of gout?
- Gout is caused by urate crystal precipitation
- What drug is used to treat gout, and how does it work?
- Allopurinol
- It inhibits xanthine oxidase, reducing uric acid levels
For IMP formation, it isn’t require aspartate rather than fumarate?
I believe you’re correct. Fixed.