Page created on April 8, 2018. Last updated on December 18, 2024 at 16:55
The complement system is a major part of the effector system of the humoral (non-cellular) immune response. It’s a part of the innate immune system but has a connection to the adaptive immune system.
It has several components. Inactive factors in the serum called complement factors will be activated and initiate an enzyme cascade, similar to the enzyme cascade of the blood clotting system. Cells have special receptors called complement receptors that bind activated components of the complement system. Lastly, the complement system has regulatory proteins that inhibit the system to prevent uncontrolled activation of it.
After the complement system has been activated, it will perform multiple functions. A complex will be formed that lyses and kills pathogens and cells. Pathogens will be opsonized by complement factors, which will make phagocytosis easier. Lastly, it will mediate inflammation.
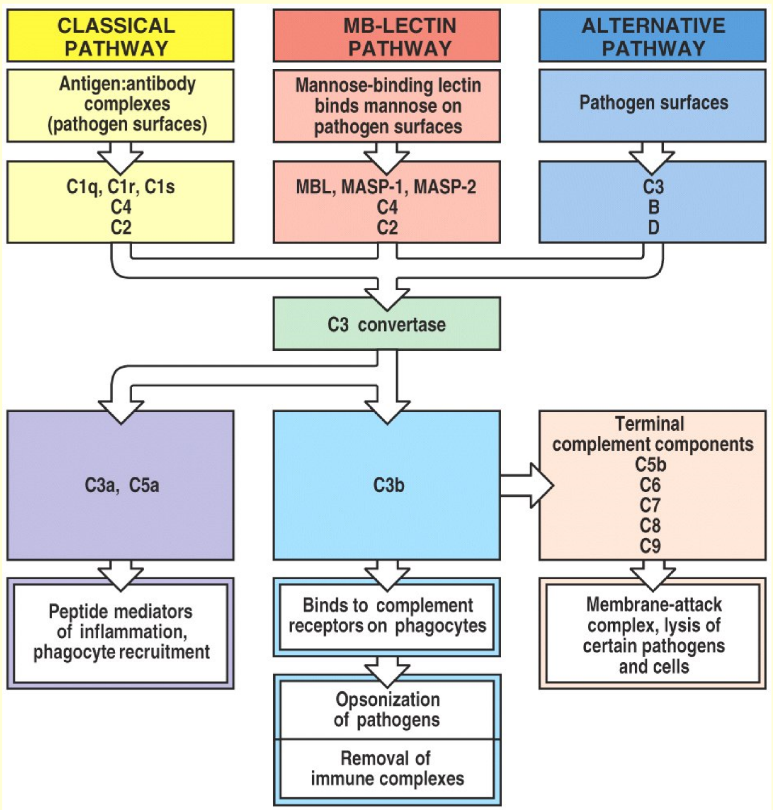
As seen on the figure to the right, the complement system can be activated in three ways, all of which result in the same result. The purpose of all three pathways is to create a large number of C3a and C3b molecules by forming a C3 convertase. You should know all the pathways.
The classical pathway is activated when a microbial cell surface is covered in either IgM or IgG1, IgG2 or IgG3. A complement protein called C1 will bind to the Fc regions of these antibodies. This activates C1, which will then cleave two other proteins, C2 and C4 into fragments C2a and C4b. These two fragments will combine into a complex called C4b2a, also known as C3 convertase. This enzyme will convert C3 into C3a and C3b. C3b will bind to the microbe to coat it (C3b is an opsonin), but it has another function as well. C3b will also combine with the C4b2a complex to yield a C4b2a3b complex, also called C5 convertase.
The lectin pathway is not activated by antibodies, but by the binding of a protein called mannose-binding lectin (MBL) to microbes. This causes cleavage of C2 and C4. The rest of the pathway will proceed like the classical pathway. The two pathways are basically identical except the lectin pathway has MBL instead of C1 and is activated by mannose on pathogens instead of antibodies.
The alternative pathway begins after C3b is present on the pathogen. There is always some free C3b found in the serum. A protein called factor D will activate another protein called factor B. C3b will then bind the activated form of factor B, called Bb (this is confusing, I know), to form the C3bBb complex. This complex also works as a C3 convertase like in the classical pathway, which will convert even more C3 into C3a and C3b. More C3b fragments will be formed, and will bind to the C3bBb complexes to form C3bBb3b complexes, which function as C5 convertases.
After C5 convertase has been formed, the cascade will continue through C6, C7, C8 and C9 until a polymer consisting of C5, C6, C7, C8 and many C9 units has been formed. This polymer is called the membrane attack complex, or MAC. This complex will create a hole in the pathogen, which will lyse it and kill it.
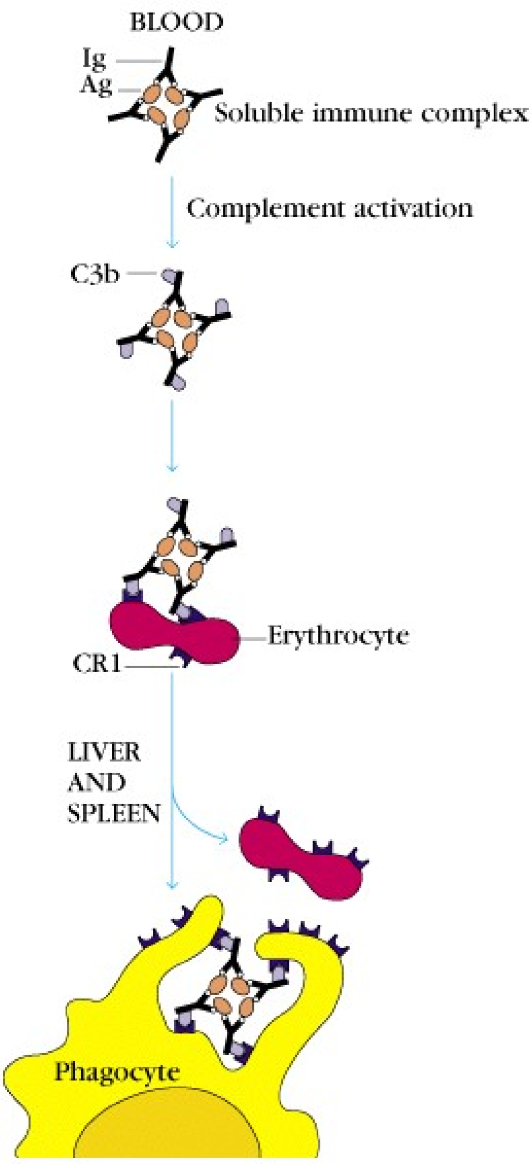
Pathogens that are coated with C3b are phagocytosed more easily, as phagocytes express complement receptor 1 (CR1), which binds C3b.
Erythrocytes also express CR1 and are therefore able to bind C3b. But they aren’t phagocytes, so why? The CR1 expression on RBCs has another purpose than phagocytosis. Because RBCs are abundant in the blood, they have an ability to bind pathogens that are covered in C3b, and transport them to the liver and spleen, where the RBCs will release the pathogens to be phagocytosed.
The smaller fragments C3a and C5a attract neutrophils and stimulate the release of inflammatory cytokines, which will increase inflammation.
Regulation of the complement system
A protein called C1 inhibitor inhibits the classical and lectin pathways. C3 convertase is inhibited by a protein called decay accelerating factor (DAF).