Table of Contents
Page created on June 5, 2019. Last updated on December 18, 2024 at 16:57
Summary
Cytotoxic drugs inhibit division of tumor cells and induce their apoptosis, usually by damaging DNA or interfering with mitosis. They’re more effective against rapidly dividing tumor cells.
Alkylating agents like cyclophosphamide alkylates DNA, which damages it. Antimetabolites like methotrexate are structurally similar to important metabolites and can be incorporated into DNA or damage DNA in other ways. Cytotoxic antibiotics like doxorubicin inhibit topoisomerase II and damages DNA by this and other mechanisms. Cytotoxic plant derivatives like vincristine impair the formation of the mitotic spindle.
Clinical application of antitumor agents
Combining multiple antitumor agents increases the cytotoxicity against cancer cells without necessarily increasing the general toxicity. It also prevents the tumors from developing resistance to individual agents.
Drugs are often given in large doses intermittently in several courses with a few weeks break between. This is to allow the bone marrow to regenerate between the courses.
When considering which drugs to combine, the cyfollowing factors are considered:
- Combining drugs with different modes of action is preferred
- Combining drugs with different toxicity is preferred
- Like choosing one drug that is less myelotoxic (like cisplatin) with a drug that is more myelotoxic
- Combining drugs which act at different parts of the cell cycle
Introduction to antineoplastic drugs
Antineoplastic agents can be divided into two subtypes:
- Cytotoxic compounds – drugs that directly damage tumor cells (and, to a lesser extent, healthy cells)
- Non-cytotoxic compounds – drugs that inhibit mechanisms needed for growth and division of tumor cells. They are less toxic
- Hormonal agents – act only on hormone-sensitive tumors
- Biological therapy – drugs that affect biological processes of tumor cells
Cytotoxic drugs
Cytotoxic drugs inhibit division of tumor cells and induce their apoptosis, usually by damaging DNA or interfering with mitosis. Their efficacy depends on the proportion of tumor cells that are actively dividing. This causes them to be more effective against fast-growing tumors.
Most of these drugs are given IV, but some can be given orally.
These drugs cannot intelligently target only tumor cells; non-tumor cells are also affected, especially those that are fast dividing like:
- GI tract mucosa
- Bone marrow
- Testicles
- Hair follicles
Due to this these drugs cause severe side effects. To prevent these side effects these drugs are often given intermittently rather than continuously.
Common side effects of cytotoxic drugs include:
- Bone marrow depression (myelotoxicity)
- Immunosuppression
- Gastrointestinal symptoms
- Nausea
- Vomiting
- Diarrhoea
- Ulceration
- Hair loss
- Growth retardation in children
- Infertility
- Nephrotoxicity
- Massive killing of tumor cells releases large amounts of uric acid, which damages the kidneys
Certain cytotoxic drugs modify DNA and can therefore actually be teratogenic and carcinogenic.
Groups of cytotoxic drugs:
- Alkylating agents – these drugs attach alkyl groups into DNA bases, which interferes with cell replication
- Alkylating-like agents (platinum compounds) – these drugs act similarly to alkylating agents
- Antimetabolites – these drugs interfere with DNA synthesis
- Cytotoxic antibiotics – these drugs inhibit the function of DNA-related enzymes
- Cytotoxic plant derivatives – these drugs mostly prevent spindle formation during mitosis
- Others
Alkylating agents
These drugs are the most commonly used cytostatic drugs.
Alkylating agent work by covalently binding alkyl chemical groups into DNA bases. This alkylation most commonly occurs on guanine (G) bases. The alkylated guanine can be excised, paired with an A or T instead of the normal C or form cross-links with other alkylated guanine bases. No matter the exact outcome, the result is that replication and transcription are inhibited, causing the tumor cell to undergo apoptosis.
Many alkylating agents are pro-drugs which are converted into an active metabolite in the body. These active metabolites are often bifunctional, meaning that they can alkylate two guanine bases and not just one.
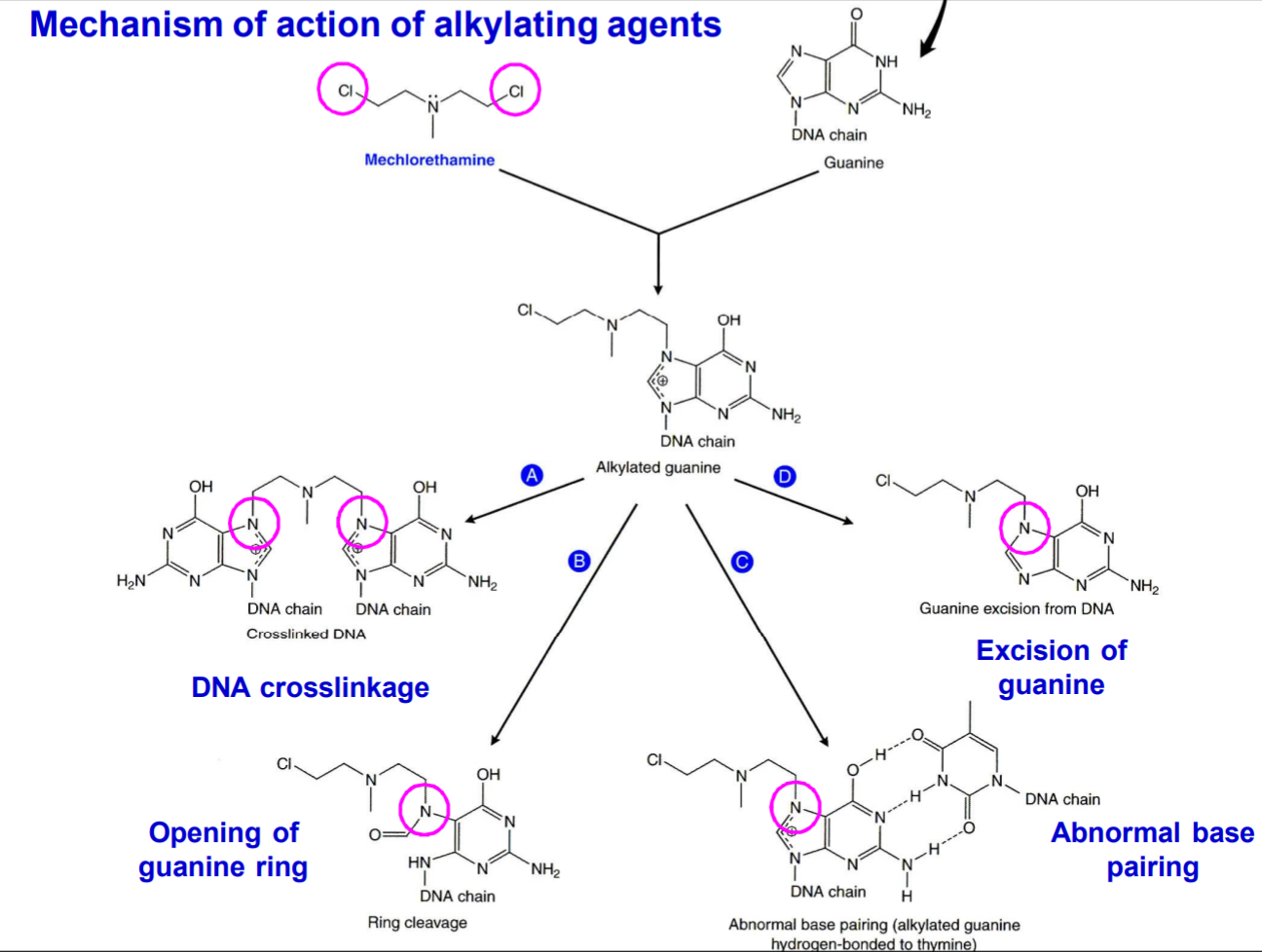
The important types of alkylating agents are:
- Nitrogen mustards
- Nitrosoureas
- Aziridines
- Others
- Busulfan
- Procarbazine
- Dacarbazine
Nitrogen mustards
Drugs of this group are related to mustard gas, a chemical warfare agent.
This drug class includes mechlorethamine, cyclophosphamide, ifosfamide, trofosfamide, chlorambucil, melphalan and bendamustine.
Cyclophosphamide is the most commonly used alkylating agent. Ifosfamide and trofosfamide are similar to cyclophosphamide.
Indications:
Cyclophosphamide is a prodrug and is used to treat chronic and acute lymphoblastic leukaemias (CLL, ALL), breast cancer and as an immunosuppressant.
Mechanism of action:
These drugs alkylate DNA at guanine bases, which inhibits DNA replication and transcription.
Dosing:
Cyclophosphamide can be given per os or IV.
Side effects:
Recent studies have determined that IV cyclophosphamide causes fewer side effects than oral cyclophosphamide.
A toxic metabolite of cyclophosphamide called acrolein causes haemorrhagic cystitis. This is also seen in ifosfamide and trofosfamide. Haemorrhagic cystitis can be relieved by giving N-acetylcysteine.
Nitrosoureas
Drugs of this group includes carmustine, semustine, lomustine and nimustine.
These drugs are lipophilic, which enables them to cross the blood-brain barrier.
Indications:
Nitrosoureas are used to treat brain tumors and metastases.
Mechanism of action:
These drugs alkylate DNA at guanine bases which inhibits DNA replication and transcription.
Side effects:
These drugs cause significant myelotoxicity.
Aziridines
Drugs of this group includes triethylenemelamine and altretamine.
Indications:
Aziridines are used to treat ovarian cancer.
Mechanism of action:
These drugs alkylate DNA at guanine bases which inhibits DNA replication and transcription.
Side effects:
These drugs cause significant myelotoxicity.
Other alkylating agents
- Busulfan
- Procarbazine
- Dacarbazine
Alkylating-like agents
The only important alkylating-like agents are the platinum compounds. These include cisplatin, carboplatin and oxaliplatin. They’re alkylating-like because they act in a similar manner to alkylating agents (both types damage DNA by forming bonds), but the alkylating-like agents don’t do it by alkylating.
Like the name suggests these compounds contain platinum. Cisplatin is inorganic while carboplatin and oxaliplatin are organic compounds.
Indications:
- Testicular cancer
- Ovarian cancer
- Bladder cancer
- Colon cancer
Mechanism of action:
These drugs don’t alkylate, but form bonds between and within strands of DNA just like alkylating agents.
Side effects:
Cisplatin is less myelotoxic than other cytotoxic drugs, but it is strongly emetogenic (induces vomiting). It is also nephrotoxic, ototoxic (toxic to the ear) and neurotoxic. A 5-HT3 receptor antagonist called ondansetron can significantly decrease the nausea.
Antimetabolites
Antimetabolites are structurally similar to substrates of intermediary metabolism. They’re typically prodrugs which are converted into metabolites that inhibit DNA synthesis.
There are multiple types of antimetabolites:
- Folate analogues – drugs similar in structure to folate
- Pyrimidine analogues – drugs similar in structure to pyrimidine bases
- Purine analogues – drugs similar in structure to purine bases
Folate analogues
Folate (folic acid, vitamin B9) itself doesn’t have any function, but it is converted (by reduction) into dihydrofolate (DHF or FH2) and then again to tetrahydrofolate (THF or FH4). Tetrahydrofolate is important in biosynthesis of purines and pyrimidines, which are important components of DNA.
THF is an important cofactor as it carries 1-carbon chemical groups like methyl groups to where they’re needed. Specifically, it is a cofactor for thymidylate synthetase, an enzyme that is required for the synthesis of dTMP, a nucleotide that forms the “T” bases in DNA.
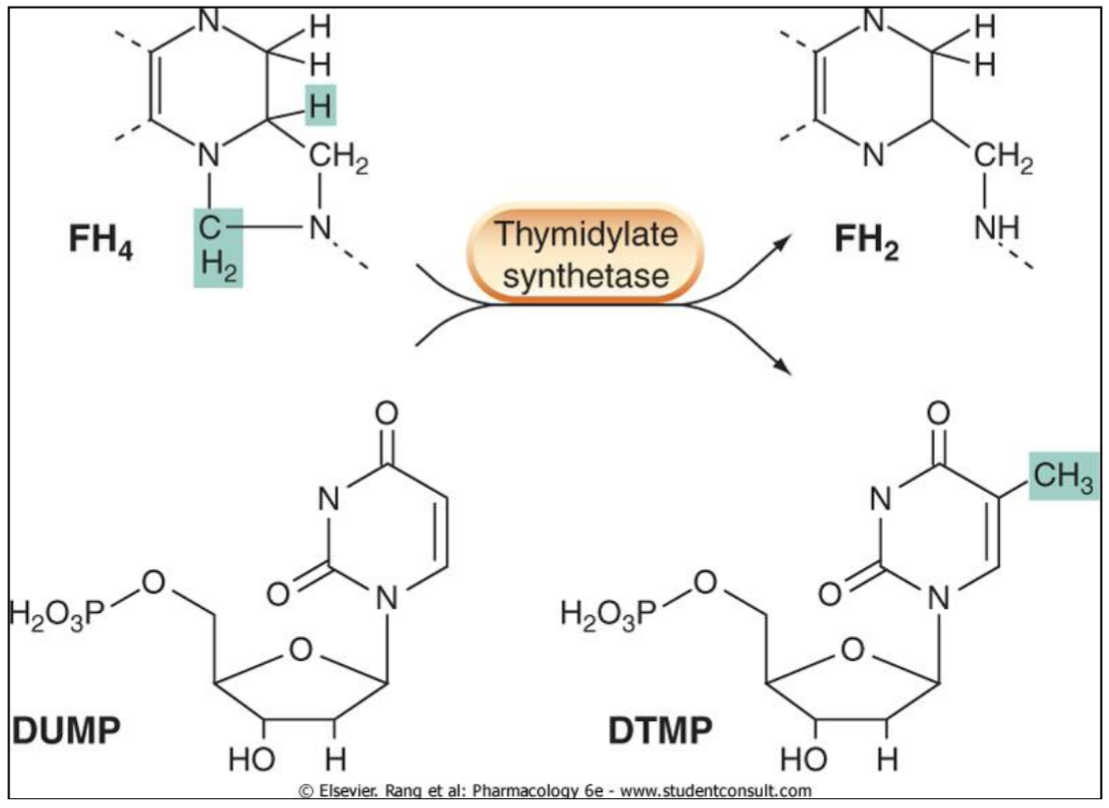
Tetrahydrofolate carries a methyl group to dUMP, converting it to dTMP.
Four folate analogues exist: methotrexate, pemetrexed, pralatrexate and trimetrexate. These drugs are structurally similar to folate, but they can’t be converted to tetrahydrofolate like folate can. This depletes the cells of THF, which decreases dTMP synthesis and therefore DNA synthesis. Methotrexate is the most important of these.
Methotrexate, pemetrexed and pralatrexate are transported into cells through the folic acid transporter. Trimetrexate is a lipophilic drug that enters cells by diffusion. After they’ve entered the cells these drugs are converted into polyglutamate derivatives. These derivatives are retained in the cells for many weeks.
Indications:
Methotrexate is used to treat choriocarcinoma, breast cancer, lymphoma and osteosarcoma. It is also used as an immunosuppressant, antirheumatic and antipsoriatic.
Mechanism of action:
These drugs act as folate and inhibit the synthesis of dTMP and therefore DNA.
Dosing:
Methotrexate can be given IV or per os. The others can only be given IV.
Side effects:
Myelotoxicity and damage to the gastrointestinal epithelium occurs. Nephrotoxicity can occur in high doses.
Antidote:
Leucovorin, also known as folinic acid, is an antidote to these drugs. It is a form of folic acid that replenishes cells with THF after a high dose of methotrexate. This decreases the side-effects.
For pemetrexed and pralatrexate folic acid and vitamin B12 can reduce the side-effects.
Pyrimidine analogues
Cytosine (C), thymine (T) and uracil (U) are pyrimidine derivatives.
The important drugs here are fluorouracil, floxuridine, capecitabine, cytarabine, gemcitabine and 5-azacytidine.
Indications:
Fluorouracil is used to treat breast cancer, skin tumors, colon cancer and liver metastases. It’s given with leucovorin (folinic acid) which boosts its effect.
Mechanism of action:
Fluorouracil (fluorinated uracil) is converted intracellularly to fluorinated dUMP (F-dUMP). Thymidylate synthetase cannot convert it into dTMP and therefore interferes with DNA synthesis.
Capecitabine is a prodrug that is converted into fluorouracil by the liver and the tumor cells.
Cytarabine is phosphorylated into cytarabine triphosphate, which is an analogue of dCTP, inhibiting DNA synthesis. Gemcitabine acts in a similar fashion.
Azacytidine is converted into azacytidine A-CTP and A-dCTP and is incorporated into DNA and RNA, inhibiting DNA synthesis.
Side effects:
Myelotoxicity and damage to the gastrointestinal epithelium occurs.
Purine analogues
Adenine (A), guanine (G) and hypoxanthine are purine derivatives.
The important drugs here are mercaptopurine, thioguanine, fludarabine, cladribine, clofarabine and pentostatin.
Indications:
Mercaptopurine is used to treat acute leukaemias (ALL, AML). It also has immunosuppressant effect.
Mechanism of action:
Mercaptopurine is a hypoxanthine analogue. It inhibits de novo purine synthesis and it is incorporated into DNA and RNA, causing damage.
Thioguanine is a guanine analogue but otherwise has the same mechanism of action as mercaptopurine.
The adenosine analogues are phosphorylated into a triphosphate form, which is incorporated into the DNA, causing damage.
Cytotoxic antibiotics
These drugs directly inhibit DNA synthesis.
The important drugs here are the four anthracyclines doxorubicin, daunorubicin, epirubicin and idarubicin, and dactinomycin, bleomycin and mitomycin.
Indications:
Doxorubicin is the most commonly used anthracycline and one of the most commonly used cytotoxic drugs overall; it’s used for many tumors. The other anthracyclines are less frequently used.
Dactinomycin is used to treat rhabdomyosarcoma, Wilms tumor and testicular cancer.
Mechanism of action:
The anthracyclines have several mechanisms of action. They bind between the bases of the DNA (called intercalation), they inhibit topoisomerase II and they form free radicals which damage the DNA. Topoisomerase II’s function is to separate the two DNA strands.
Dactinomycin is also intercalated into the DNA which inhibits transcription and replication.
Bleomycin binds to DNA and iron, forming reactive oxygen species that damage the DNA.
Mitomycin has two mechanisms of action. It produces reactive oxygen species like bleomycin, but it is also an alkylating agent.
Side effects:
Anthracyclines cause significant myelotoxicity, but they are also cardiotoxic, causing arrhythmias and heart failure. The cardiotoxic effect can be limited by giving iron chelators like dexrazoxane.
Bleomycin and mitomycin may cause pulmonary fibrosis.
Cytotoxic plant derivatives
Three types of cytotoxic plant derivatives are used:
- Vinca alkaloids
- Taxanes
- Topoisomerase inhibitors
The vinca alkaloids are derived from catharanthus roseus. The important drugs of this group are vincristine, vinblastine and vinorelbine.
The taxanes are derived from trees of the taxus species. The important drugs of this group are paclitaxel, docetaxel and cabazitaxel.
The topoisomerase inhibitors are derived from various plants, but common for all of them is that they inhibit topoisomerase I or II. They are etoposide, teniposide, topotecan and irinotecan.
Indications:
Vincristine is used to treat ALL, lymphomas and paediatric tumors.
Vinblastine is used to treat CLL and lymphomas.
Mechanism of action:
The Vinca alkaloids inhibit polymerization of tubulin into microtubules, which prevents the formation of the mitotic spindle. This prevents cell division.
The taxanes work in a similar way. They actually stimulate polymerization, but this also inhibits normal function of the mitotic spindle and prevents cell division.
Topotecan and irinotecan inhibit topoisomerase I, while etoposide and teniposide inhibit topoisomerase II. The result of either of these is that replication and transcription are blocked and breakage of the DNA strands occurs.
Side effects:
Vincristine is primarily neurotoxic, causing neuropathy.
Vinblastine is highly emetogenic and myelotoxic.
Vinorelbine is myelotoxic.
Paclitaxel is neurotoxic, and it might trigger hypersensitivity reactions.
Topotecan and irinotecan can cause severe diarrhoea.
Other cytotoxic agents
These agents don’t fit in any other groups.
Hydroxyurea inhibits ribonucleotide reductase and therefore DNA synthesis.
Iodine-131 is a radioactive isotope of iodine. Like normal iodine it is taken up by follicular cells in the thyroid, but unlike normal iodine it releases β-radiation. It’s used to treat thyroid cancer.
Mitotane is selective for cell in the adrenal cortex, which is why it’s used to treat metastatic adrenocortical tumors.
Amsacrine inhibits DNA topoisomerase II. It has cardiotoxic effect.
Mitoxantrone induces breakage of DNA strands.
Asparaginase is an enzyme that is used to treat ALL. It is selective for leukaemia cells as these cells have a large demand for asparagine, and the enzyme decreases the level of the amino acid. This drug is not myelotoxic.
Drugs used to relieve the side effects of cytotoxic therapy
Cytotoxic therapy carries many side effects. Some of these can be limited with the help of other drugs.
- Nausea and vomiting can be treated with
- 5-HT3 receptor antagonists, so-called “setrons”
- Dopamine antagonists
- Dexamethasone
- Myelosuppression can be treated with
- Haematopoietic growth factors
- Haematopoietic stem cell transplant
- Hyperuricaemia can be treated with
- Allopurinol
- Uricosuric drugs
- Uricolytic agents
Hello Greek Doctor!
I found a sentence that kinda doesn’t make sense, (Alkylating agents like cyclophosphamide alkylates and damages DNA), cyclophosphamide alkylates and (what ?) Damages DNA.
Thank you for your continuous work !
Alkylates here is a verb in 3rd person present form, not a plural noun. Cyclophosphate “performs alkylation” on DNA, damaging it.
I’ll change the wording to make it less ambigous.