Table of Contents
Page created on February 22, 2019. Last updated on December 18, 2024 at 16:57
Peptic ulcer
Peptic ulcers are mucosal breaks that penetrate the mucosa and may penetrate into the submucosa. They’re usually in the stomach or duodenum. Duodenal ulcers are mostly associated with too much acid while gastric ulcers are mostly associated with decreased protective factors.
Both types are associated with alcohol, NSAID use, stress and most importantly, helicobacter pylori. H. pylori is responsible for the vast majority of peptic ulcers. It’s diagnosed based on the urea breath test, where the patient ingests radioactively labelled urea. H. pylori has a urease enzyme which breaks down urea into ammonia and bicarbonate. Some of the bicarbonate is exhaled as CO2. The amount of radiolabelled CO2 can be measured.
The main symptom is pain. Duodenal ulcers usually cause pain when fasting which improves upon eating, while gastric ulcers cause pain that becomes worse when eating. Bleeding may also occur.
Peptic ulcers are treated conservatively with drugs. There are many types of drugs available to treat the disease. First we must look at the mechanisms of which acid is secreted and how the mucosa is protected from it.
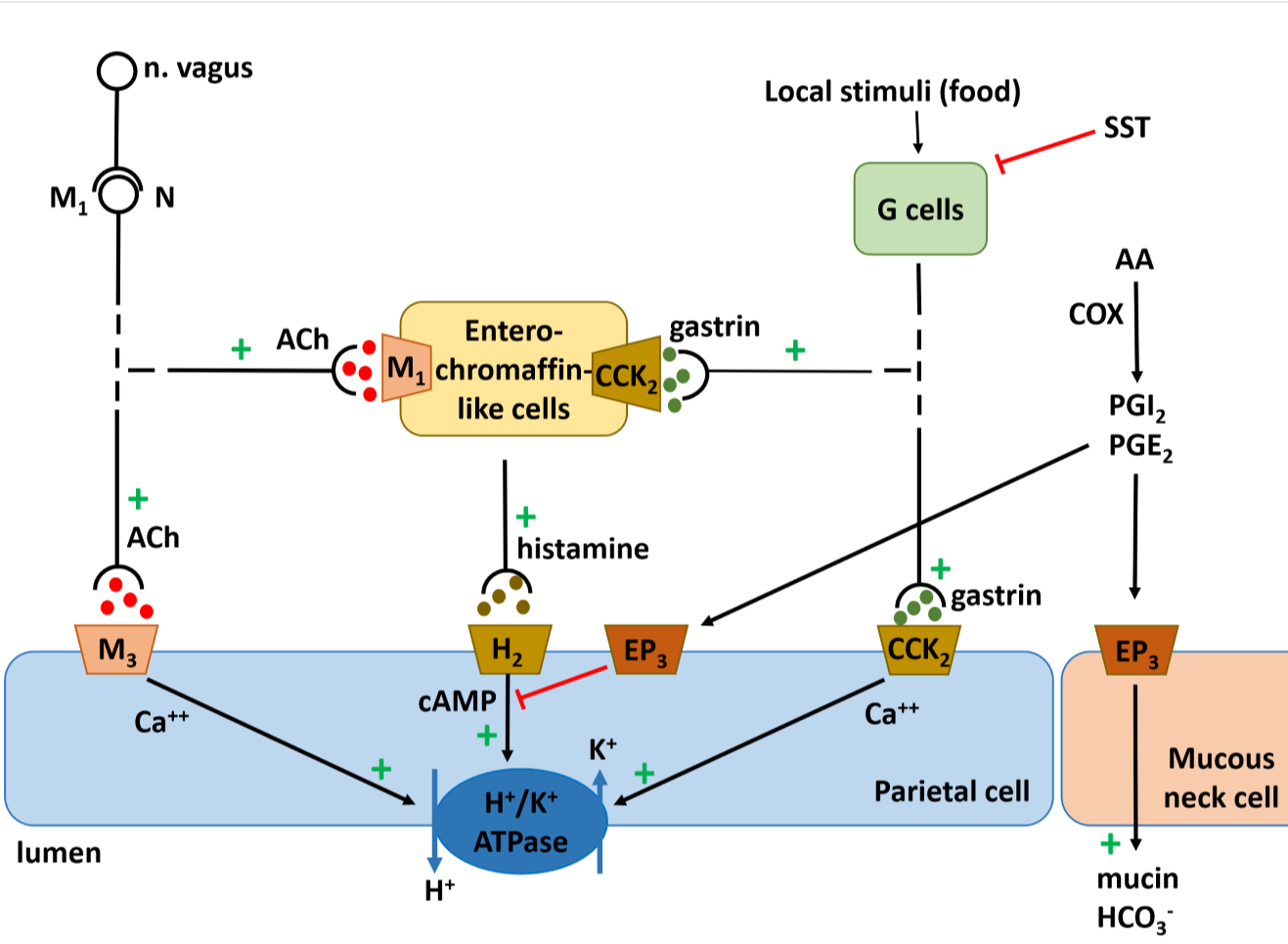
The mechanisms that regulate gastric acid production.
The HCl component of stomach acid is produced by the H+/K+ ATPase, also called the proton pump, inside parietal cells. As you can see from the figure are there many factors that influence the activity of the proton pump. This means that there are also many possibilities for drugs to regulate gastric acid production.
Proton pump inhibitors
These drugs, often called PPIs, inhibit gastric acid production by the easiest mechanism: by binding to and inhibiting the proton pump itself. The prototype is omeprazole.
Compounds:
- Omeprazole
- Esomeprazole
- Pantoprazole
- Lansoprazole
Indications:
- GERD
- Peptic ulcer
- Zollinger-Ellison syndrome
- Acid reflux
Mechanism of action:
PPIs bind to the H+/K+ ATPase and inhibit it irreversibly. These drugs are lipophilic weak bases that are inactive at neutral pH. Because they are lipophilic can they cross the cell membrane of parietal cells and enter them. The environment inside the parietal cells is highly acidic. This causes the PPIs to take up H+ from the environment and become protonated. They are now no longer lipophilic as they have a positive charge, so they are “trapped” inside the parietal cells.
Dosage:
These drugs are given orally inside enteric-coated granules that prevent them from being trapped inside the stomach. The drugs are released inside the intestines, where they are absorbed and travel with the blood to the parietal cells of the stomach. They should be taken in the morning.
Side effects:
- GI symptoms
- Increased risk of C. difficile infection
- Decreased iron and B12 absorption
- Long term:
- Decreased absorption of calcium
- Osteoporosis
Histamine 2 receptor antagonists
Compounds:
- Cimetidine
- Ranitidine
- Famotidine
Indications:
GERD, peptic ulcer. Prophylaxis against stress ulcer.
Mechanism of action:
These drugs block the histamine 2 receptor which normally stimulates gastric acid secretion. These drugs not only decrease acid secretion but also pepsin secretion. They promote ulcer healing.
Pharmacokinetics:
H2 receptor antagonists are eliminated by renal excretion.
Side effects:
- Cimetidine
- Erectile dysfunction
- Gynecomastia
Muscarinic antagonists
M1-selective muscarinic antagonists like pirenzepine and telenzepine block the neuronal regulation of gastric acid production. These drugs are only used for ulcers that don’t respond to PPI or H2 blocker therapy.
These drugs have parasympatholytic side-effects like bronchodilation and decreased secretions.
Antacids
Antacids are weak bases which forms salts with HCl in the stomach, neutralizing the acid. Most antacids that are used are salts of both magnesium and aluminium. Magnesium salts cause diarrhoea and aluminium salts cause constipation, but by combining them can we preserve normal bowel function.
Compounds:
- Systemic antacids
- Sodium bicarbonate – NaHCO3
- Sodium citrate
- Non-systemic antacids
- Magnesium hydroxide – Mg(OH)2
- Aluminum hydroxide – Al(OH)3
- MgO
Mostly non-systemic antacids are used today.
Indications:
Symptomatic treatment for GERD, heartburn. Non-systemic antacids are preferred.
Mechanism of action:
Antacids are salts of bases which will neutralize gastric acid.
Antacids are classified as either systemic or non-systemic, the difference being whether they’re absorbed by the GI tract or not. Those that are non-systemic are not in water soluble form in the basic pH of the small intestine, so they are not absorbed. The systemic ones are absorbed as they are in water soluble form in basic pH, so they can cause metabolic alkalosis and are therefore not preferred.
Counterindications:
Usage of aluminum and magnesium-containing antacids in people with renal failure causes hyperaluminaemia and hypermagnesaemia.
Interactions:
Because they alter the pH may they decrease the absorption of drugs like digoxin, anticoagulants and antibiotics.
Side effects:
Bicarbonate forms CO2 when it meets the acid in the stomach, which may cause discomfort and flatulence. Because it’s a systemic antacid it can also cause metabolic alkalosis.
It’s important to keep in mind that development of milk-alkali syndrome may occur in antacid administration. The syndrome develops when a person consumes too much calcium and alkali. All antacids are alkali, so people shouldn’t drink milk when using antacids.
Mucosal protectants
These drugs enhance endogenous mechanisms that protect the mucosa or they provide a physical protective barrier for the mucosa.
Sucralfate is a substance that creates a thick gel-like protective layer on the mucosa and especially on the ulcers. This drug needs acidic pH to work and should therefore not be used with acid-lowering drugs. It’s mostly used as a prophylactic of stress-induced ulcers.
Bismuth compounds are compounds with the ion bismuth (Bi3+). Bismuth has bactericide effect against H. pylori and forms a protective layer on the mucosal surface. May be used together with antibiotics against the bacterium. Very little bismuth is absorbed but like for aluminium it can cause encephalopathy in renal failure. Rarely used as they cause black discoloration of the teeth and tongue.
Misoprostol is a prostaglandin E1 analogue. Prostaglandin E1 has protective effects on the mucosa. It decreases acid secretion, increases mucous and HCO3– secretion and increases mucosal blood flow. It’s used to prevent NSAID-related ulcers in long-term NSAID therapy. Diarrhoea and nausea are very common side-effects.
Carbenoxolon is derived from the liquorice plant. It increases gastric mucus production and decreases acid production. It’s not frequently used due to its mineralocorticoid-like side-effects.
Antibacterial treatment
The go-to treatment of H. pylori-positive peptic ulcer, the so-called “triple therapy” consists of 7 days treatment with:
- A proton pump inhibitor
- Clarithromycin
- Amoxicillin or metronidazole
A so-called quadruple therapy involves bismuth as well.
Proton pump inthibitor not protein pump hehe :))
Jesus, and I misspelled like three times too.. and no one noticed haha
Side effect of muscarinic antagonist are bronchodilaton?
Actually, you’re right? I just fixed it? Thanks?
Chuckled a bit at this.