Table of Contents
Page created on December 1, 2018. Last updated on December 18, 2024 at 16:56
Congenital heart disease
Congenital heart disease (CHD) are anomalies of the heart and/or the great vessels that are present from birth. They’re the result of faulty embryogenesis in the first trimester. Some form of CHD occurs in 1 in every 100 births, and 12 disorders account for 85% of all CHDs. The occurrence of the most important is shown in the table below:
| Malformation | % of all CHDs |
| Ventricular septal defect | 42 |
| Atrial septal defect | 10 |
| Pulmonary stenosis | 8 |
| Patent ductus arteriosus | 7 |
| Tetralogy of Fallot | 5 |
| Coarctation of aorta | 5 |
There are several causes of CHD, mostly genetic or environmental. Most causes are sporadic. The non-sporadic causes are are:
- Genetic
- Trisomy 13, 15, 18, 21
- Turner syndrome
- Down’s syndrome (trisomy 21) is the most common cause of CHD
- Environmental
- Rubella
- Teratogens
- Alcohol
Three types of congenital heart diseases exist. They are:
- CHDs with left-to-right shunts
- Ventricular septal defects (VSD)
- Atrial septal defects (ASD)
- Patent ductus arteriosus (PDA)
- Atrioventricular septal defect (AVSD)
- CHDs with right-to-left shunts
- Tetralogy of Fallot
- Transposition of the great vessels
- Truncus arteriosus communis
- Tricuspid atresia
- Total anomalous pulmonary venous connection
- Obstructive CHD
- Coarctation aortae
- Pulmonary stenosis
- Aortic stenosis
Congenital heart defects with left-to-right shunts
Left-to-right shunts range from asymptomatic to complete heart failure. Sometimes can asymptomatic left-to-right shunts develop into fatal conditions, so it’s important to screen for them. Especially ASDs tend to be asymptomatic, so in most places are infants screened for ASDs.
Left-to-right shunts aren’t as dangerous as right-to-left shunts from an oxygenation perspective. The system blood isn’t less oxygenated when blood flows from the left side of the heart to the right side. However, the shunt causes blood that have already passed through the pulmonary circulation once to do so again, so the pulmonary blood flow and pressure is increased. This damages the pulmonary circulation, causing fibrosis, which leads to pulmonary hypertension.
In any kind of left-to-right shunt, but especially in VSDs Eisenmenger syndrome can develop. Recall that the pressure inside the left ventricle is much higher than in the right ventricle. With a shunt between the two (or between the atria) will blood flow from the LV into the RV during systole with high pressure. This exposes the right ventricle to high levels of stress, to which it responds by hypertrophying. After this process has worked over a long period of time can the right ventricle hypertrophy so much that the pressure inside the right ventricle exceeds that of the left ventricle. This effectively reverses the shunt from left-to-right to right-to-left!
When the shunt reverses is the patient said to have Eisenmenger syndrome. Now, as with any right-to-left shunt, is deoxygenated blood shunted from the right ventricle into the left ventricle and into the systemic circulation, causing cyanosis and hypoxic organ damage. The kidney senses the hypoxia and produces erythropoietin, which causes polycythaemia which increases the viscosity of the blood. At this point is the lung damage so significant that even if the CHD is surgically corrected is the only treatment for the patient a lung transplant.
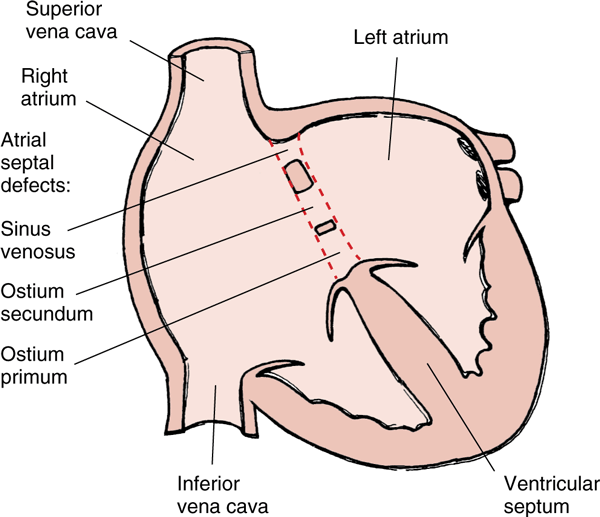
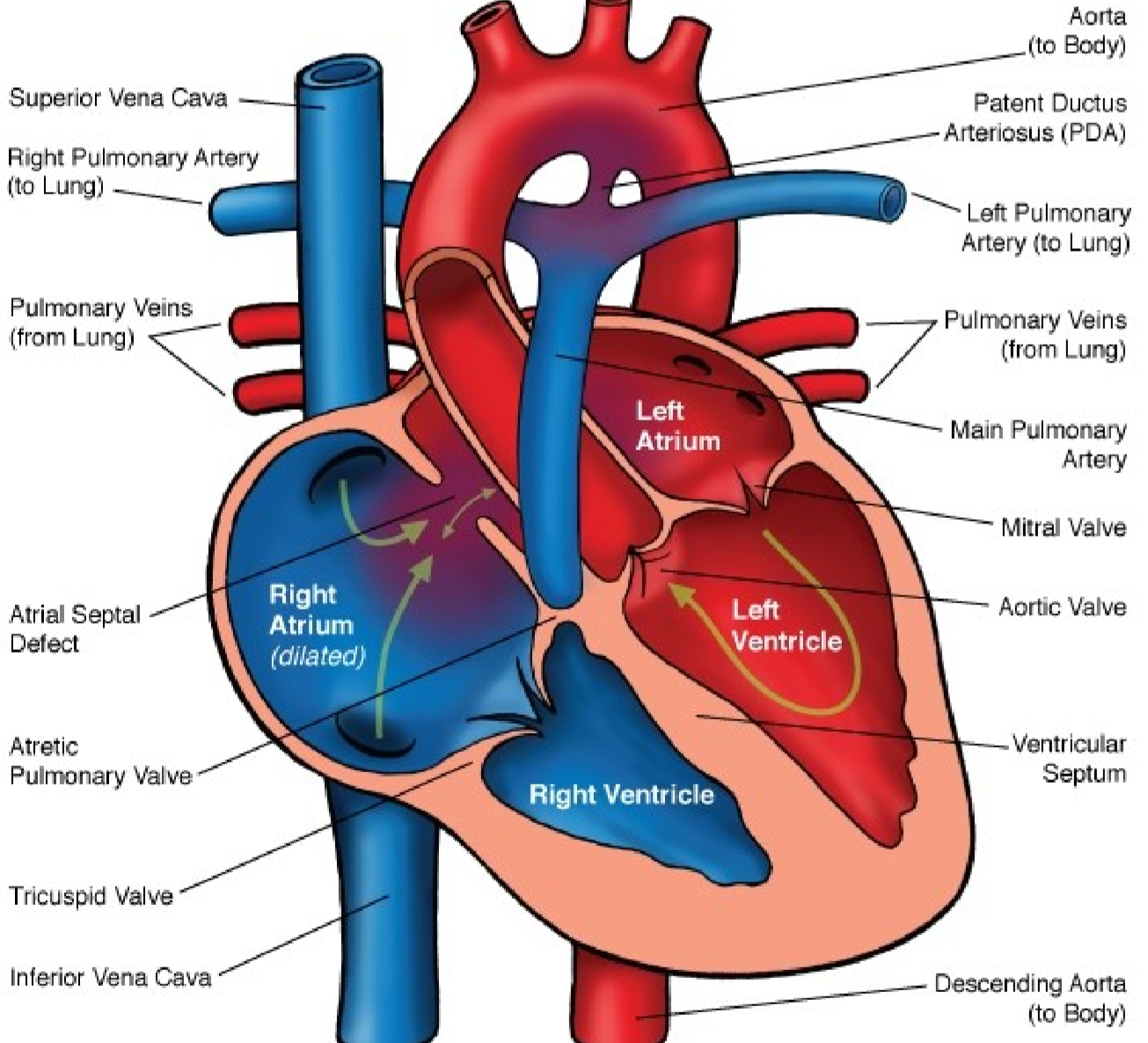
Atrial septal defects exist in multiple types. Recall from embryology that the atrial septum is made up of two tissue flaps, septum primum and septum secundum. 90% of all ASDs occur due to the septum secundum not properly closing the ostium secundum. These ASDs are called septum secundum ASDs. The two other types of ASDs are septum primum ASDs and sinus vensosus ASD, together account for just 10% of all ASDs.
- Septum secundum ASD – 90% of cases
- Septum primum ASD
- Sinus venosus ASD
In 20% (!) of the population the foramen ovale never closes during embryonic development and stays open forever. This condition is called patent foramen ovale (PFO) and can be seen as an ASD. PFO is mostly asymptomatic, however it does allow for something called paradoxical embolization, where venous emboli can enter the systemic circulation by passing through the PFO instead of going into the pulmonary circulation.
ASDs are mostly asymptomatic until the age of 30. Hypertrophy of the right ventricle and dilatation of the right atrium may occur to compensate for the increased right-sided circulation.
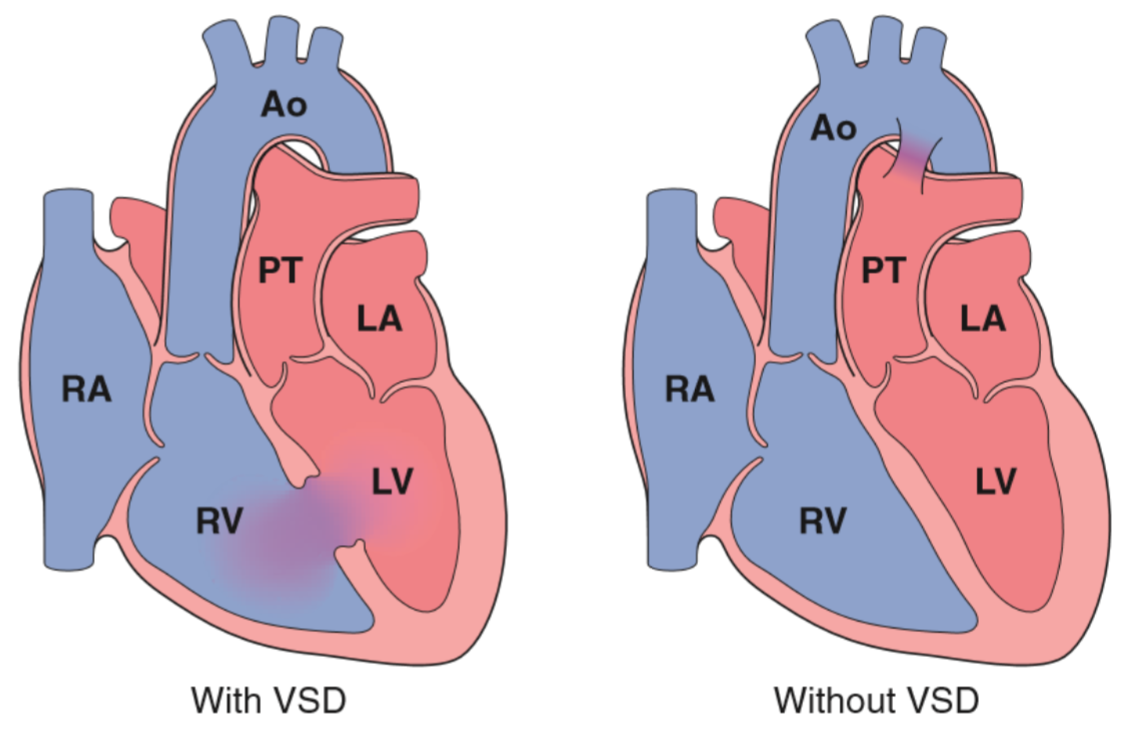
Ventricular septal defects exist in just two types. Recall again from embryology that the ventricular septum is made up of two types of septa, the membranous septum and the muscular septum. Defects in the membranous septum is called Roger disease and account for 90% of all VSDs, while defects in the muscular septum accounts for just 10% and don’t have a cool name.
VSDs are the most common type of CHD. It occurs isolated in 30% of cases but is most commonly associated with other CHDs, such as Tetralogy of Fallot. VSDs are rarely asymptomatic, only small ones are, but the larger ones result in severe left-to-right shunting, which can cause pulmonary hypertension, congestive heart failure and Eisenmenger syndrome. Small or medium VSDs don’t shunt as much blood as large ones, however they “jet” a small beam of blood onto the wall of the right ventricle with each heartbeat, which can damage the endothelial lining of the RV. This can increase the risk for development of infective endocarditis.
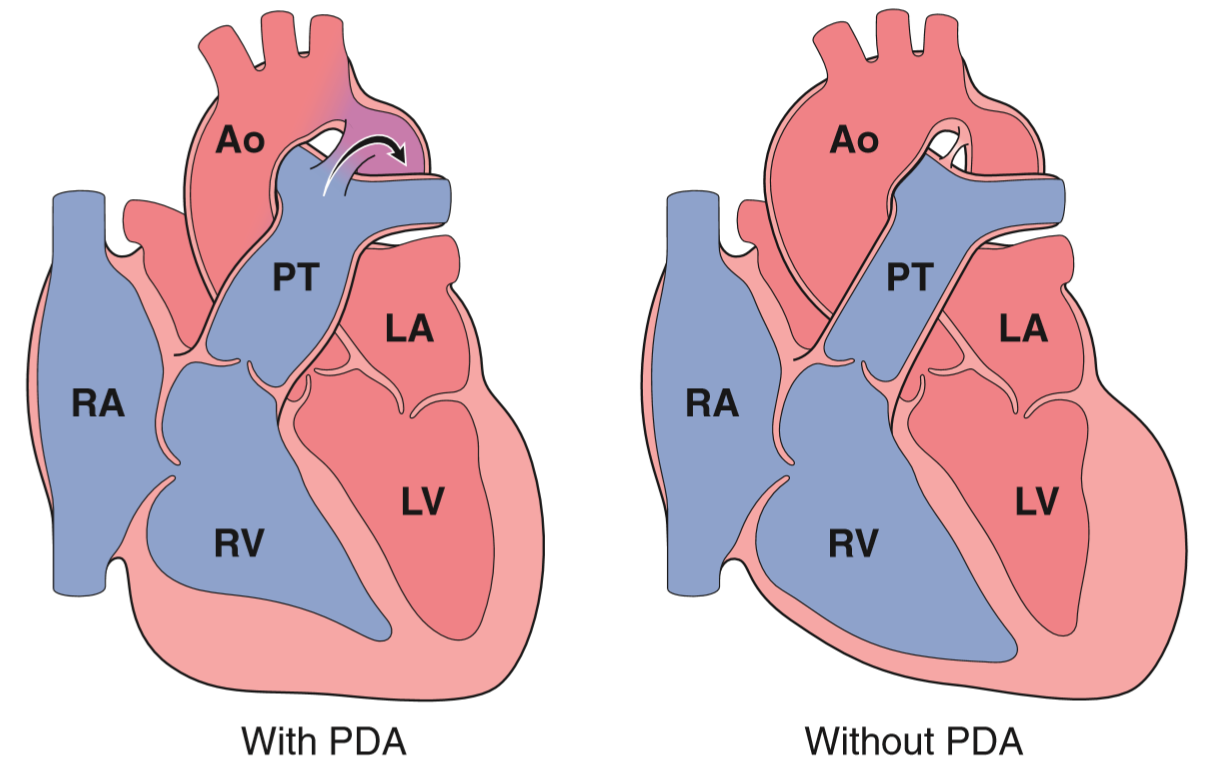
Patent ductus arteriosus (= ductus Botalli persistens as the pathology department wants to call it), occurs when the ductus arteriosus between the pulmonary trunk or artery and aorta never closes. It usually closes within one week after birth. Because the pressure inside the aorta is much higher than in the pulmonary circulation the blood will flow from the aorta into the pulmonary circulation, so this is also a left-to-right shunt.
In 90% of cases it occurs isolated, and in only 10% is it associated with other CHDs. They can be small enough to be asymptomatic, or they can lead to Eisenmenger syndrome.
Atrioventricular septal defect (AVSD) occurs when there is no atrioventricular septum at all. It occurs when the superior and inferior endocardial cushions fail to fuse. Two types exist: incomplete AVSD, which is actually a septum primum ASD with abnormal anterior mitral cusp, and complete AVSD, where all four chambers communicate freely.
Congenital heart defects with right-to-left shunts
In right-to-left shunts deoxygenated blood will be directed into the systemic circulation, which causes hypoxaemia and most notably, cyanosis.
Tetralogy of Fallot is the most important one. It’s a tetralogy because it involves four abnormalities:
- Ventricular septal defect
- Right ventricular outflow tract obstruction (subpulmonic stenosis, narrowing of the infundibulum)
- Overriding aorta
- The aorta originates right over the VSD, so blood from the right ventricle enters the aorta during systole
- Right ventricular hypertrophy
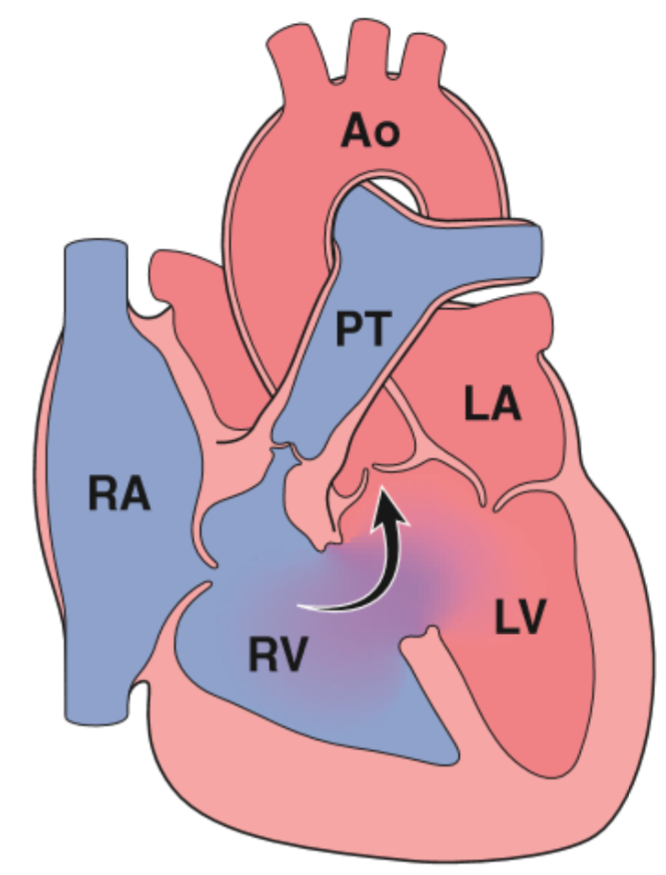
The heart is large and boot-shaped. Because of the subpulmonic stenosis the pulmonary trunk issmaller than normal, while the proximal aorta is dilated. The pulmonary blood flow is decreased because of the stenosis, and the clinical severity depends largely on how stenotic the outflow tract is. If it is mildly stenotic will the symptoms be similar to a VSD, but if it’s severely stenotic will cyanosis develop early. 10% of untreated patients are still alive at 20 years, and only 3% at 40 years.
While the child develops and grows will the demand for oxygen increase, but the pulmonary stenosis stays the same, so the symptoms become progressively worse. Common symptoms are cyanosis and polycythaemia.
Transposition of the great vessels occurs when the aorta arises from the right ventricle and the pulmonary trunk from the left ventricle. This causes the systemic and pulmonary circulations to be completely separated, which logically, is incompatible with life, except in the cases where a shunt between the two circulations exists. This allows blood to be mixed, which provides some oxygenated blood to the systemic circulation.
VSD, patent foramen ovale and patent ductus arteriosus are all common shunts that can keep a person with transposition alive, at least for a while. VSDs are stable shunts, but PFOs and PDAs commonly close after birth. These infants need immediate surgical intervention within the first few days of life.
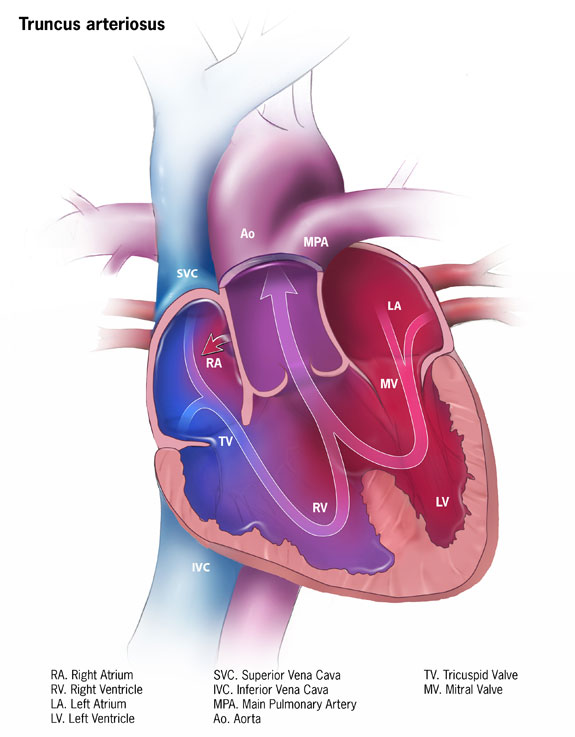
Truncus arteriosus communis is the failure of separation of the truncus arteriosus into the aorta and pulmonary trunk. The result is that there is one arterial trunk that arises from both ventricles and provides blood to both the pulmonary and systemic circulation. The consequences are early systemic cyanosis and pulmonary hypertension.
Obstructive congenital heart defects
Coarctation of the aorta is the name for a congenital narrowing of the aorta. There are two types, the infantile, or preductal form and the adult or postductal form.
The infantile form occurs when there is also a patent ductus arteriosus present, and the PDA opens distally to the coarctation. This causes the majority of the blood in the aorta to come from the pulmonary trunk, which is deoxygenated. Cyanosis develops, and most affected infants don’t survive the neonatal period without surgery.
The adult form occurs without a PDA. They’re mostly asymptomatic, and the disease may remain unrecognized until well into adult life. Usually can the patient have upper extremity hypertension and lower extremity hypotension.
Pulmonary atresia is the third most common CHD. An atresia is an abnormal stenosis or closing of an orifice. It occurs with a mild to severe stenosis of the pulmonary valve. It can occur isolated, or part of other CHD like Fallot and transposition of great vessels.
The right ventricle will be hypertrophied to compensate for the stenotic valve. The pulmonary trunk (after the stenosis) will be dilated because of the jetstream blood flow that comes through the stenotic valve.
In some cases can the pulmonary valve be completely atretic, where absolutely no blood flows through it. This is only compatible with life if the patient also has a PDA and an ASD. The right ventricle will be hypoplastic, and blood will only flow into the lung through the PDA from the aorta. Blood flows from the right side of the heart into the left side through the ASD.
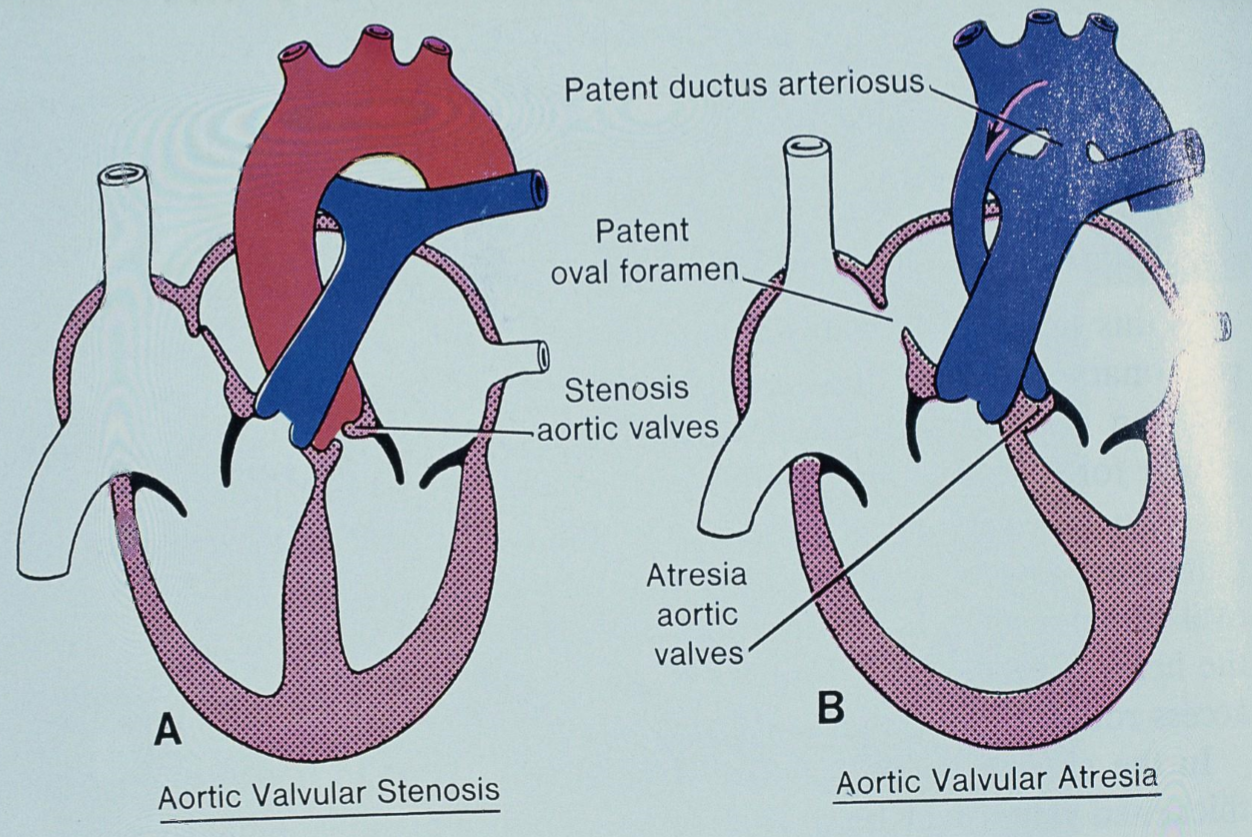
Aortic atresia exists in three types:
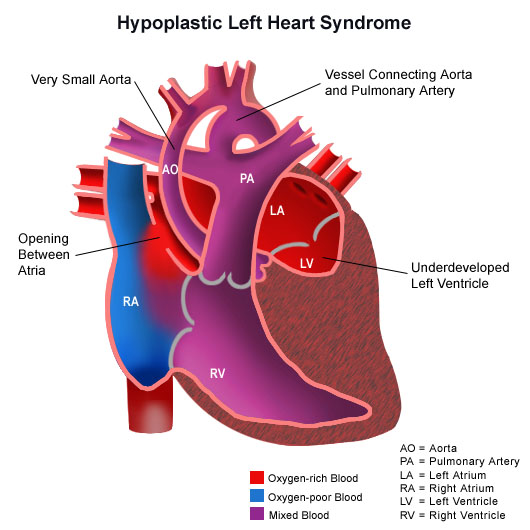
- Valvular type occurs when the valve is hypoplastic or thickened. This is only compatible with life if the patient also has a patent ductus arteriosus and an ASD or a VSD, so that the aorta can be supplied from the right ventricle. The aortic valve will be severely stenotic or completely atretic, which renders the left ventricle useless, causing it to be hypoplastic. The ascending aorta is hypoplastic as well, because it receives little blood flow. This condition is called hypoplastic left heart syndrome, and can be fatal if the ductus arteriosus closes after birth (which it normally does).
- Subaortic type occurs when there’s dense endocardial fibrous tissue below the valve. The valve will be stenotic, but still open.
- Supravalvular type is an inherent form of aortic dysplasia, where the ascending aortic wall is thickened, causing the lumen to be obstructed. This occurs in Wiliams syndrome.
Both the subaortic and supravalvular types are associated with concentric left ventricular hypertrophy.