Table of Contents
Page created on March 14, 2018. Last updated on December 18, 2024 at 16:55
How do immune cells meet the antigen?
It’s important that the immune system has the correct reaction to an antigen, but for the immune system to do anything it has to know that there are foreign antigens present. If you cut your finger and only a few bacteria enter the wound (or survive the disinfectant you apply), it seems very unlikely that they will be recognized by an immune cell in a short period of time.
There are two ways for the initial antigen-recognition to happen. The antigen can, in dissolved free form, be drained by the lymph to a lymph node where it will be recognized. The other way happens more commonly. Dendritic cells present in skin (Langerhans cells) and mucosa (no special name) regularly patrol their tissues, scanning them for anything that might be suspicious. If a dendritic cell finds anything, it will phagocyte it, degrade it and present it on MHC II (recall that dendritic cells are antigen-presenting cells and therefore express MHC II). Inactive dendritic cells in the periphery are immature until they phagocyte an antigen. When they do, they will mature and express B7, and will then migrate to a lymph node, where the appropriate immune cell will eventually find it.
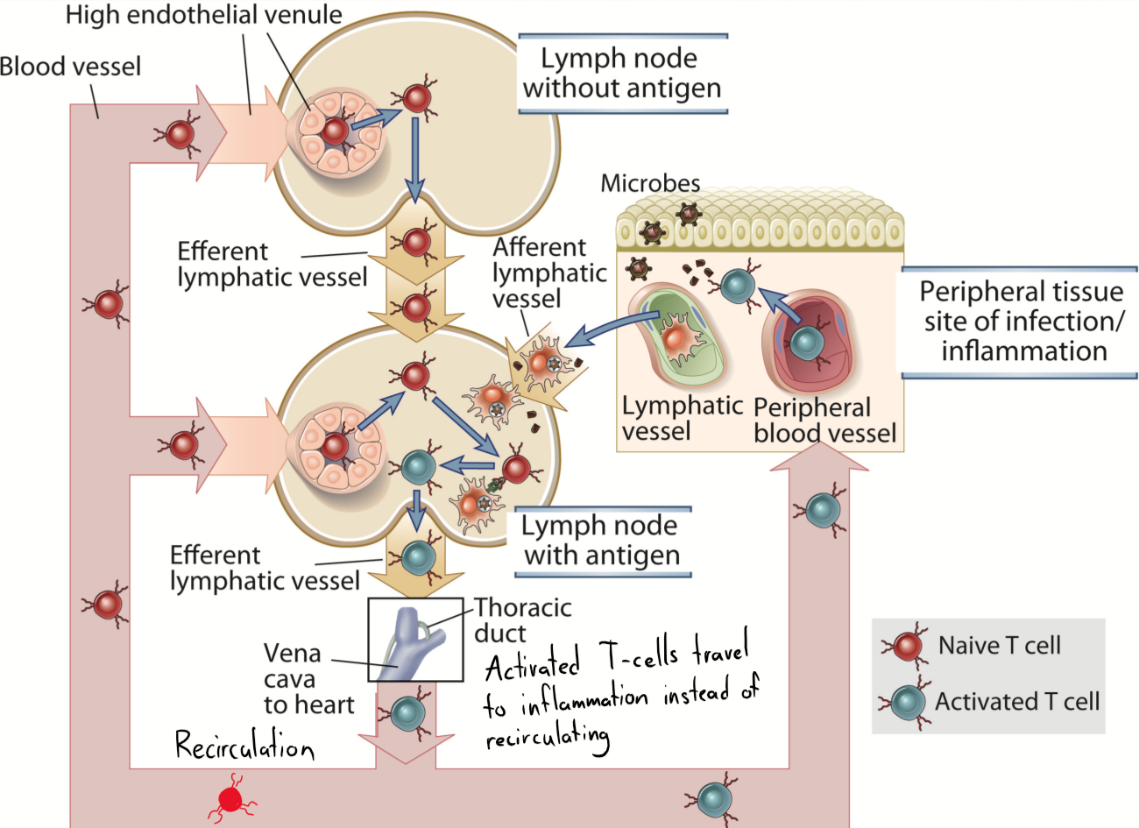
A naïve T-cell lives approximately 3 weeks. These T-cells travel with the blood to a lymph node, which they will “scan” by moving around inside it and seeing if anything binds to their TCR. If not, they will eventually leave the lymph node through its efferent lymphatic vessel to enter another lymph node. It will continue this until it reaches the thoracic duct, from which it will travel with the blood to a distal lymph node again, repeating process. However, if the T-cell actually does bind an antigen in the lymph node, it will get activated, and will leave the lymph node and enter the blood. However, instead of recirculating to lymph nodes, it will travel to the site of inflammation where the antigen first entered.
An activated T-cell will start expressing CCR7 and L-selectin, both which are important for its migration to the inflamed tissue. The endothelium close to the inflamed tissue will express integrins and ICAM-1, which the T-cell will bind to, which lets it know that it has reached the inflamed tissue.
T-cell activation
The process when a T-cell first encounters the antigen presented by an APC is called priming. During this priming, the T-cell and APC bind through various adhesion molecules, which initiate 2 signals inside the T-cell that make it differentiate and proliferate into effector and memory T-cells. The first of these signals is the binding of the TCR-CD3 complex on the T-cell to MHC II on the APC. This signal is antigen-specific, as the binding wouldn’t happen if the appropriate antigen wasn’t present in the MHC II. The second signal is the binding of CD28 on the T-cell to B7 on the APC.
Both signals are needed for successful activation. If only one the B7 – CD28 signal is present, nothing will happen (because this binding happens between every APC and every T-cell, regardless of whether antigens are present or not). If only the MHC II – TCR/CD3 signal is present, the T-cell will be inactivated.
The immunological synapse
Some of the proteins that are embedded on the surface of cells can move around on the surface. These cell surface proteins are embedded into some special parts of the cell membrane that are more rigid. These rigid parts are called lipid rafts. You can imagine the cell surface proteins as humans riding on a raft on the ocean. The “raft” is the lipid raft, a rigid part of the cell membrane, and the “ocean” is the rest of the cell membrane, that the lipid raft floats upon. These lipid rafts, along with the proteins sitting on them, can float around on the cell surface to move around. The cytoskeleton helps this lipid raft to move.
Both the T-cell and the APC have their surface proteins like MHC II, TCR/CD3 complex and CD28 spread mostly evenly among the cell surface. However, when priming first occurs, the surface proteins of both cells will move around on the cell surface (thanks to the lipid rafts), so that some of the surface proteins will cluster around a small point (centrally), while others migrate to a create a “ring” surrounding the central surface proteins (peripherally), which yields a bull’s eye-like appearance of the surface proteins (see the image below). These clusters are supramolecular activation complexes, or SMACs, and are basically complexes of multiple surface proteins involved in the priming.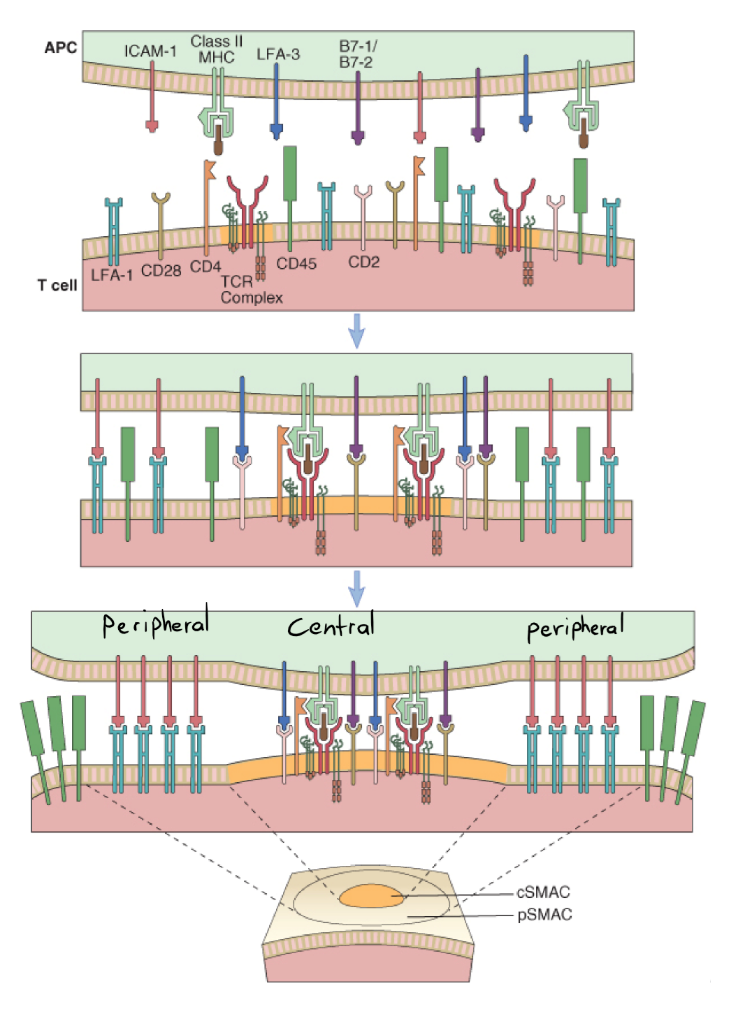
The formation of the immunological synapse. Note how some surface proteins move to the middle, while some create a circle around the middle. Looks like a cracked egg actually. cSMAC is central supramolecular activation complex, while pSMAC is peripheral SMAC. Don’t make me SMAC you
TCR signalling
After something binds the TCR, the receptor has to propagate the signal into the cell. However, the TCR itself doesn’t contain any domains that can do this, however CD3 can. Activation of TCR by binding something will cause CD3 to propagate the signal. This is why TCR is found in a complex with CD3 on the cell; they work together.
After TCR has bound its ligand, tyrosine residues on the intracellular part of CD3 will be phosphorylated. Two proteins called Lck and Fyn will be activated, which in turn activate a protein called ZAP-70. This protein will activate many proteins, like PLC, the MAPK cascade and transcription factors like NF-κB, NF-AT and others.
These transcription factors increase the transcription of many proteins. Examples include IL-2 to IL-6 and other cytokines. Some proteins are transcribed immediately, some first after a few hours, and some after days or weeks.
T-cell activation
What happens after the T-cell is activated? It will start to proliferate, to differentiate and start expressing CTLA-4.
Naïve T-cells express just a few IL-2 receptors, which also have just moderate affinity for IL-2. However, when activated, the T-cell will start expressing many of these receptors, but now with a high affinity instead. The cell also produces IL-2, which will bind to the high-affinity receptors and cause the T-cell to proliferate. This is an autocrine mechanism.
After a T-helper cell (CD4+) is activated, it will differentiate into one of 4 types (actually 6 but we focus on 4). They’re summarized in the table below. The transcription factors are probably not too important to know.
|
Type of Th |
Induced by: | Important transcription factor | Produces the following cytokines | Present against these infections | Important in what disease |
| Th1 | IL-12 | T-bet | IL-1, TNF, IFNγ | Viruses, intracellular bacteria | Autoimmune disease, chronic infection |
| Th2 | IL-4 | GATA-3 | IL-4, IL-5, IL-6, IL-13 | Parasites | Allergy |
| Th17 | TGFβ, IL-6, IL-21, IL-23 | RORγt | IL-17 | Extracellular bacteria, fungi | Organ-specific autoimmunity |
| Treg | TGFβ, IL-2, retinoic acid | FoxP3 | IL-10, TGFβ | Regulates immune response | ? |
Lastly, activation of the T-cell causes it to express more CTLA-4. Recall that both CTLA-4 and CD28 bind to B7 on antigen-presenting cells, but CTLA-4 binds to with more affinity than CD28 does. While CD28 bind to B7 to activate the T-cell, CTLA-4 binds to B7 to inactivate it. When CTLA-4 is activated, it will send inhibitory signals to the activated T-cell, causing it to “calm down”. This mechanism controls the immune response and makes sure the immune system isn’t sent into overdrive.