Table of Contents
Page created on April 19, 2018. Last updated on December 18, 2024 at 16:55
Differentiation of CD8+ T-cells to cytotoxic lymphocytes
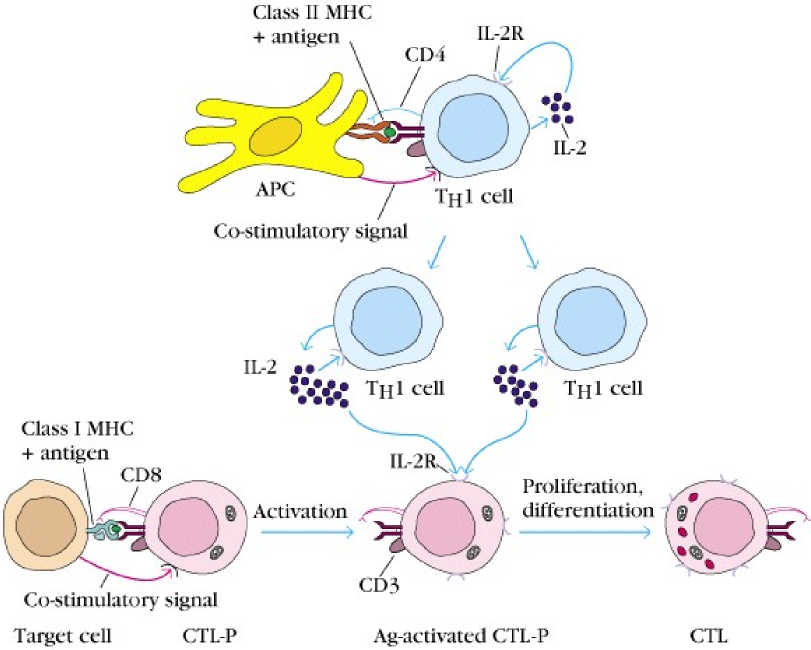
Naïve CD8+ need to be activated by an antigen before it can really be cytotoxic and kill other cells. As we know by now, CD8+ T-cells can only recognize antigens when they are presented by a cell through an MHC I molecule.
Naïve CD8+ cells are also called cytotoxic lymphocyte precursor, or CTL-P cells. After a CTL-P binds an antigen, it will be activated. This will cause it to express IL-2 receptor. Parallelly to this activation, a Th1 cell has been activated by an APC, which will cause the Th1 to start secreting IL-2. The binding of these IL-2 to the IL-2 receptor on the activated CTL-P cell will cause it to differentiate into the mature cytotoxic lymphocyte, or CTL cell.
Some of these CTL cells will become effector cells, while some become memory cells, waiting for the antigen to show up later in life. When the body is later infected by the same pathogen with the same antigen, the memory CTL cells will be activated. Memory CTL cells can produce IL-2 by themselves and therefore do not need stimulation by Th1 cells to be activated, so they can be activated quicker than the normal CTL cells.
Effector mechanisms of CTL cells
After a CD8+ has been activated to become a CTL cell, it can start killing target cells. When the CTL binds to an infected cell or a tumor cell (we call it the target cell from now on), it will form tight adhesions with the target cell with the use of adhesion molecules like integrins, creating a so-called conjugate. This causes the granules in the CTL to rearrange to the side of the CTL that faces the target cell. The CTL will then degranulate, releasing their granules onto the target cell by exocytosis.
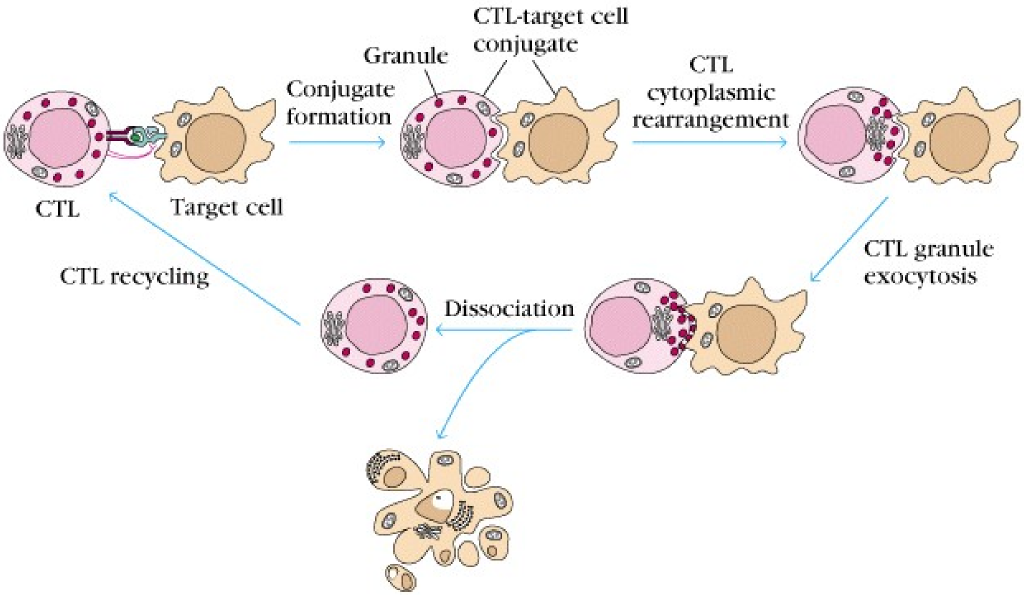
The granules contain molecules like perforin and granzymes. Perforin is an enzyme that creates a pore in the membrane of the target cell. Granzymes will then enter the target cell and kill it in two different ways. Granzyme B will activate a protein cascade called the caspase cascade (which begins with a protein called caspase 8) which is the extrinsic apoptotic pathway. Caspase 8 will activate an enzyme called CAD (caspase activated deoxyribonuclease), which cleaves DNA. This kills the cell. Granzyme A will cause DNA-damage by a different pathway, which will also induce the death of the cell.
During this process, a protein called Fas ligand will bind to the Fas receptor on the target cell, which also activates the extrinsic apoptotic pathway by activating caspase 8, resulting in the same mechanisms as by granzymes.
γδ T-cells
The γδ T-cells are similar in function to the CTL. They constitute 5% of the total T-cell count and are found in the epidermis and epithelium. They’re produced in the embryonic life. They are CD4– and CD8-.
They don’t need antigens presented to them by MHC, so they can bind antigens directly through their γδ TCR. The antigens they usually bind are viral proteins with their TCR and bacterial lipids through the surface molecule CD1.
The main function of the γδ T-cells is to kill infected epithelial cells. The killer mechanism is similar to the one in CTL cells.
NK cells
When NK cells are activated, they secrete INFγ. They’re part of the early response to infection of certain viruses, intracellular bacteria and tumor cells. They have two unique receptors called killer inhibitory receptor (KIR) and killer activator receptor (KAR). These receptors are very important in how NK cells recognize abnormal cells. The NK cells kill cells in the same way CTLs do.
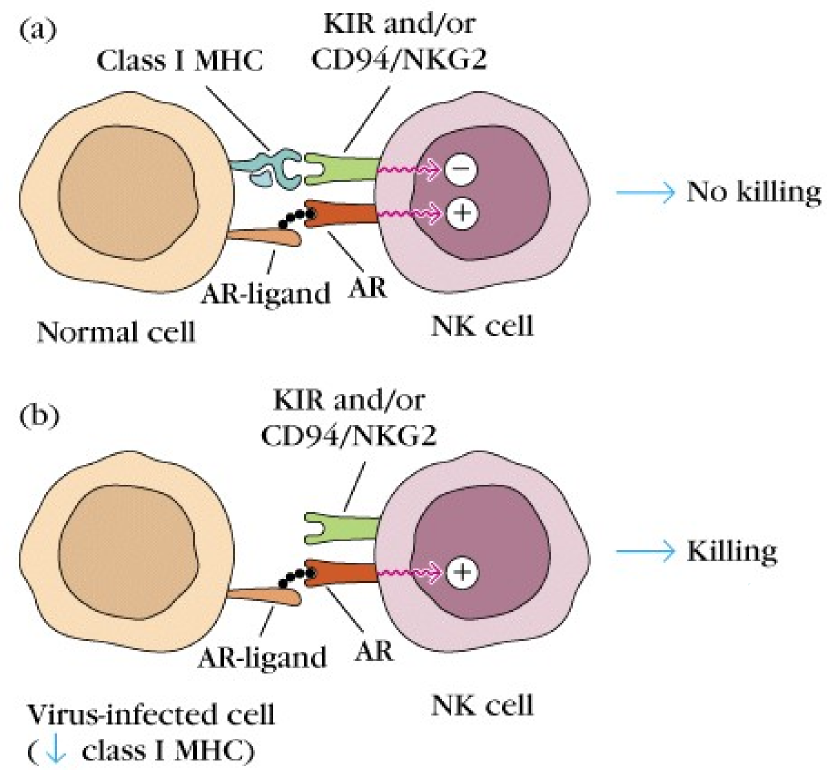
The ligand of the KIR is the MHC class I molecule (just the molecule, not the antigen presented on it!), while the ligand of the KAR is another protein on the cell surface which we’ll call KAR ligand. The KIR will, like the name suggest, inhibit the killing activity of the NK cell while the KAR will activate this activity. The inhibiting activity of the KIR is stronger than the activating activity of the KAR.
When the NK cell meets a healthy cell, the KIR on the NK cell will bind to the MHC I on the healthy cell, and the KAR on the NK cell will bind to the KAR ligand on the healthy cell. Because the activity of KIR is stronger than KAR, the net result is that the killing activity of the NK cell is inhibited, so the NK cell will just move on.
However, virus-infected cells or tumor cells often have abnormal or absent MHC I molecules on their surface. When KIR cannot bind to these cells because MHC I is abnormal or absent, there is no inhibitory signal to the NK cell. However, the KAR still binds KAR ligand on the target cell. Since there is now only an activating signal and no inhibiting signal, the NK cell will kill the target cell.
NKT cells
A special type of leukocyte called Natural Killer T cells, or NKT cell, can be found in the tissue. They’re called this because they share properties with both T cell and NK cells, as they have both CD56 and TCR. They recognize microbial phospholipids and glycolipids when presented to them by other cells through a special surface protein called CD1d.
They’re able to produce certain cytokines very quickly, like IL-4, INFγ, IL-17 and TNFα.
Brief, clear, well understandable description.
Thank you! That’s what I’m going for