Table of Contents
Page created on March 14, 2018. Last updated on December 18, 2024 at 16:55
In the bone marrow
All T-cells start as hematopoietic stem cells (HSC) in the bone marrow. The HSC can differentiate into either the common myloid progenitor (CMP) or the common lymphoid progenitor (CLP). The presence of IL-7 will stimulate the differentiation of HSC to CLP. CLP will then differentiate into pro-T cells, which will migrate to the thymus.
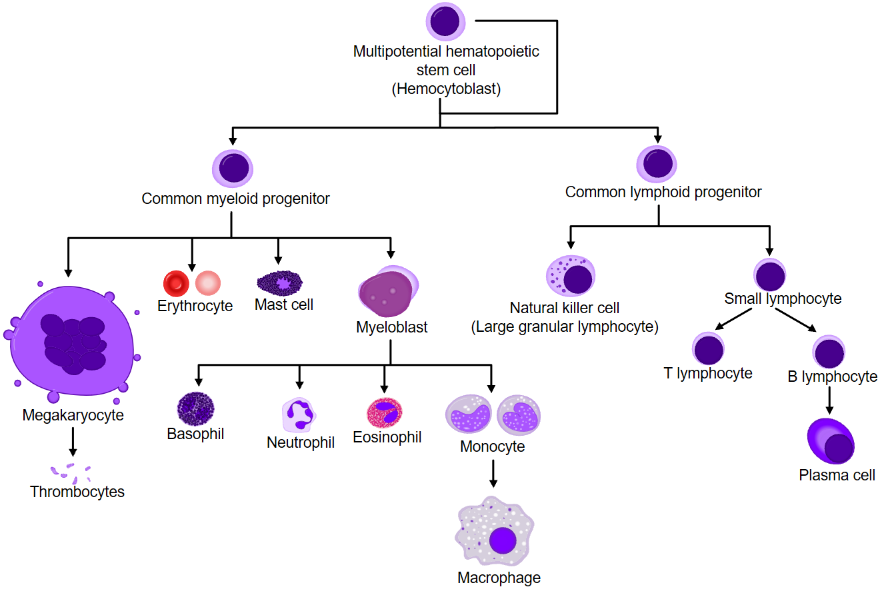
In the thymus
The pro-T cells have not yet recombined the gene for their TCR (recall the VDJ recombination). The pro-T cell will differentiate into the next developmental stage, the pre-T cell.
The pre-T cell stage has recombined the gene for the β-chain of the TCR and is expressing the pre-T cell receptor on its cell surface. The pre-T cell receptor is comprised of the finished β-chain with a “surrogate” α-chain called Tα. This α-chain is not the same as the α-chain of the finished TCR. If the pre-T cell fails to present the pre-T cell receptor, the VDJ recombination of the β-chain has failed, and the cell dies by apoptosis. This is called beta-selection. During this stage, IL-7 is needed for the development. Allelic exclusion also happens during this stage. The pre-T cell is also known as the double negative T-cell, because it expresses neither CD4 nor CD8.
After beta-selection, the cell will start expressing both CD4 and CD8, which will make it a double positive (DP) T-cell. The name comes from the fact that it is “positive” for both CD4 and CD8, because they are both present. At this stage, VDJ recombination of the α-chain is also complete, and the TCR is finished. The double positive T-cell is found in the inner cortex of the thymus. Here, the cortical thymic epithelial cells will present MHC I and MHC II with self-peptides. If the TCR on the DP T-cell can bind moderately to either MHC I or MHC II on the cortical thymic epithelial cells, the cell will live and continue development. If the cell cannot bind to either MHC I or MHC II however, it will die by apoptosis. This is called positive selection.
Recall that there are three genes for MHC I and three for MHC II on each chromosome, and that there are two of each chromosome (one from daddy and one from mommy). In total, this is 6 MHC I and 6 MHC II genes per cell. The cortical thymic epithelial cell, or CTEC for short, presents all 12 of these MHC molecules, which is important for the positive selection.
The double positive T-cell now migrates to the medulla of the thymus, where it will meet medullary thymic epithelial cells. These cells express a unique transcription factor called AIRE. This factor allows the cell to produce proteins from (almost) every organ in the body. The medullary thymic epithelial cells will then present short peptides from these proteins in MHC molecules to the double-positive T-cells. Because these peptides are found naturally in the body, they are self-antigens and the T-cells shouldn’t bind strongly to them. If they do, they are killed by apoptosis. This process is called negative selection. This selection ensures that no T-cell that can bind to self-antigens will survive and is important in having the immune system tolerate the host body.
If the DP T-cell bound to MHC I during the positive selection, it will lose the CD4 (so that it becomes a CD8+ T-cell), and if it bound to MHC II, it will lose the CD8. The T-cell is now single positive for either CD8 or CD4, depending on which MHC it can recognize. The single positive T-cell is then released into the blood, so it can travel to the periphery.
The Treg cells
If a CD4+ binds to MHC II with a slightly “too strong” affinity, but not strong enough to be eliminated by negative selection, it will differentiate into a special type of T-cell called a T regulatory cell, or Treg. These cells work as a negative feedback to the immune response by inhibiting the effect of T-cells. These Treg cells are CD25++ (they express many CD25), FOXp3+ and CTLA4+. Recall that CTLA4 is an inhibitory adhesion molecule.
Two types of Treg cells exist. The ones mentioned here, that are differentiated in the thymus are called natural Treg, or nTreg cells. The other type is called induced Treg or iTreg cells. The induced Treg cells do not become Treg cells in the thymus, but are actually CD4+ in the periphery that differentiate into Treg cells in the periphery. Their differences are illustrated in the table below.
| Characteristic | nTreg | iTreg |
|---|---|---|
| Develop where? | In the thymus | In the periphery |
| Requires costimulation by which protein? | CD28 | CTLA-4 |
| Recognize what types of antigen? | Self-antigen | All types of antigen |
| Role in immunological tolerance? | Yes | Yes, bigger role |
The γδ T-cells
The γδ T-cells are a special type of T-cells that are mostly found in tissue and not in blood. They are double negative (CD4- and CD8-) and are mostly synthesized during the embryonic stage, before birth. After 18 days of gestation, their number decrease while the number of αβ T-cells (the “normal” T-cells) increase. The level of γδ T-cells is very low in adults. The T-cell receptor of γδ T-cells is comprised of γ and δ chains instead of the normal α and β chains.
These T-cells are special because they don’t need antigens presented to them through MHC to be activated, like αβ T-cells do. They are probably important in recognizing lipid antigens.
About how DP T cell become single positive, should it be during negative selection?
I’ve skimmed the text but can’t find any mistakes, although it’s been long since I learned this and so you might be correct. Where specifically do you mean?