Table of Contents
Page created on March 14, 2018. Last updated on December 18, 2024 at 16:55
Learning objectives
- What are the body’s sources of amino acids?
- What are the two steps of amino acid metabolism?
- Which amino acids are mostly degraded elsewhere than in the liver?
- What is an aminotransferase reaction?
- Which aminotransferase reactions are important?
- What is the function of PLP as a co-enzyme?
- What are the fates of the amino group?
- How is most ammonium removed from peripheral tissues?
- What is the alanine cycle?
Metabolism of amino acids
All amino acids are comprised of an amino group and a carbon skeleton. During metabolism of amino acids these two parts are separated, as they have different “fates” in the body. The amino group can be used to synthesise new biomolecules, or it can be excreted The carbon skeleton will be used for energy, as we’ll see in topic 13.
Most amino acids are metabolised in the liver, but some are almost exclusively metabolised in muscle. This includes the branched-chain amino acids leucine, isoleucine, and valine.
Amino acids are metabolised in two steps:
Moving the amino group from the amino acid to glutamate
Splitting the amino group from the carbon skeleton is done by aminotransferases, sometimes called transaminases. Aminotransferases are enzymes that catalyse the switching of an amino group with a keto group between to molecules. This transfers the amino group from an amino acid to an α-ketoacid.
Often, these aminotransferases move the amino group from the amino acid to α-ketoglutarate. The former becomes a keto acid, while the latter becomes glutamate.
There are two aminotransferases which are important, alanine aminotransferase (ALT or ALAT) and aspartate aminotransferase (AST or ASAT).
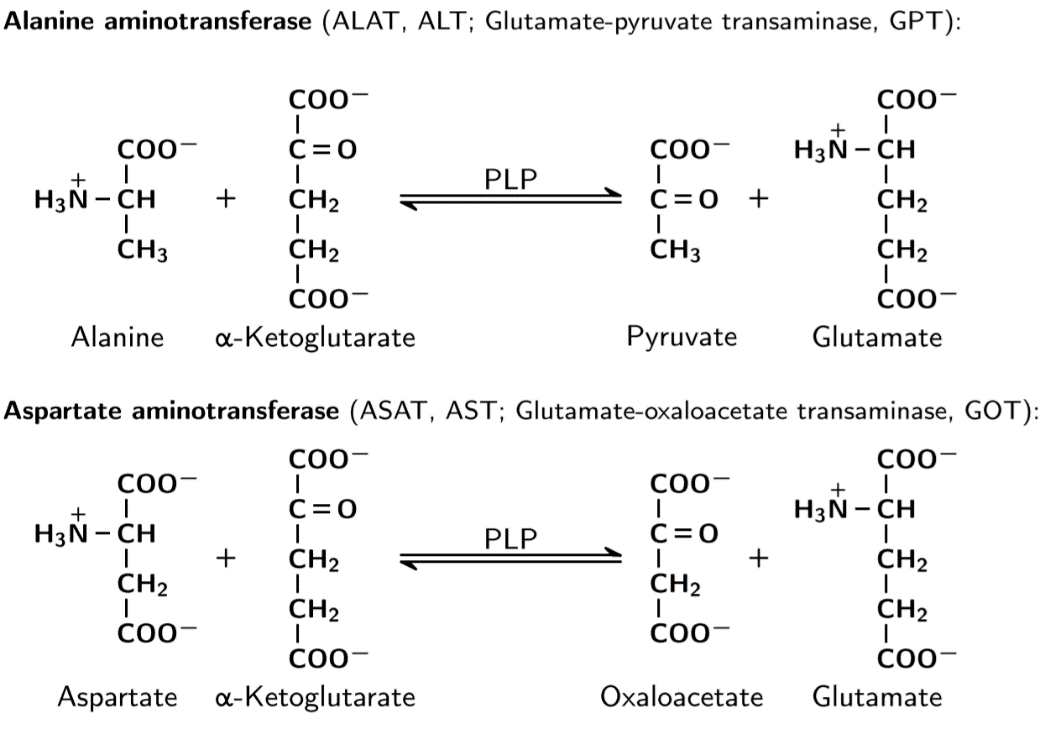
The two most important aminotransferases.
Aminotransferases are found in most cells, but are abundant in the liver. In fact, high concentration of aminotransferases in the blood is a sign of liver damage, due to the contents of the hepatocytes being released into the circulation.
Aminotransferase reactions need PLP, a cofactor derived from vitamin B6, pyridoxine. The PLP cofactor accepts and donates amino groups in chemical reactions. This is accomplished by a ping-pong reaction mechanism.
Removing the amino group from glutamate
Now that the amino group has been moved from the amino acid to glutamate, it remains to remove the amino group from glutamate. The glutamate dehydrogenase reaction is a form of oxidative deamination. It catalyses glutamate -> α-ketoglutarate + NH4+. This reaction is unusual in that it can use both NAD+ or NADP+ as cofactors.
Some amino acids don’t undergo aminotransferase at all; they just have their amino groups removed directly. This includes threonine and serine, which are nonoxidatively deaminated by threonine dehydratase and serine dehydratase, respectively. These enzymes also require PLP.
Fates of the amino group
The amino group (now in ammonium form) has multiple fates:
- It can be excreted through the urea cycle
- It can be used to synthesise new biomolecules
- Biosynthesis of amino acids
- Biosynthesis of nucleotides (topic 17)
- Biosynthesis of biological amines
- Histamine
- Dopamine
- Melatonin
- etc.
Ammonium released from amino acid metabolism in the liver can be directly excreted through the urea cycle, but not only the liver metabolises amino acids. How is ammonium transported from peripheral tissues to the liver for excretion? It can’t be through the blood, as ammonium is toxic and acidic.
Instead, glutamine synthetase combines glutamate and ammonium to produce glutamine. Glutamine is not toxic and so can be transported in the blood. Once in the liver the glutaminase reaction, which only occurs in liver mitochondria, converts glutamine back to glutamate and ammonium. The ammonium can then enter the urea cycle. Most ammonium in the body is removed this way.
The urea cycle is covered in the next topic.
The alanine cycle
Ammonium from muscle can be transported to the liver by another pathway too, the glucose-alanine cycle. The alanine cycle is similar to the Cori cycle, except this doesn’t take place in RBC’s as it depends on alanine aminotransferase (ALT), which is only present in muscle and the liver.
When muscles metabolise proteins and amino acids for energy, they release ammonium. This ammonium is incorporated into glutamate.
During muscle work glucose is also converted to pyruvate. ALT converts pyruvate and glutamate into alanine and α-ketoglutarate. Alanine is transported in the blood to the liver, where the alanine is converted back to pyruvate and glutamate by reversing the ALT reaction. Pyruvate is then used as a substrate for gluconeogenesis. The ammonium can then be released from glutamate and enter the urea cycle.
Summary
- What are the body’s sources of amino acids?
- Metabolism of intracellular proteins, or by digestion of dietary proteins
- What are the two steps of amino acid metabolism?
- 1 – transamination, which moves the amino group to α-ketoglutarate, forming glutamate
- 2 – removal of the amino group from glutamate
- Which amino acids are mostly degraded elsewhere than in the liver?
- Branched-chain amino acids are mostly degraded in muscle
- Leucine, isoleucine, valine
- What is an aminotransferase reaction?
- An aminotransferase reaction moves the amino group of an amino acid to an α-keto acid
- Which aminotransferase reactions are important?
- Alanine aminotransferase (ALT)
- Aspartate aminotransferase (AST)
- What is the function of PLP as a co-enzyme?
- PLP accepts and donates amino groups
- What are the fates of the amino group?
- Excretion via urea
- Biosynthesis of new biomolecules
- How is most ammonium removed from peripheral tissues?
- By glutamine synthetase -> glutamine to liver -> glutaminase
- What is the alanine cycle?
- Ammonium produced in muscle is converted to glutamate
- ALT converts glutamate and pyruvate to alanine and α-ketoglutarate
- Alanine transfers to the liver, where ALT is reversed
- Pyruvate is used for gluconeogenesis
- The amino group from glutamate is excreted through urea
May i ask you what are the amino acids not among 20 protein building amino acid ??
I’m not sure I understand your question. Could you rephrase it?
I have come across many questions of Biochemistry, but these types of questions are very helpful and so great.
Glad you like them!
Hi! huge fan of your work. keep it up!!
Do wanna take a look at your summer, question #2 answer 1-
Glad you like it!
I don’t know what you’re hinting to specifically, but I rewrote it to be more clear. Is it better now?