Table of Contents
Page created on March 14, 2018. Last updated on December 18, 2024 at 16:55
Learning objectives
- How are proteins targeted for the various organelles?
- Describe protein targeting to ER
- Describe protein targeting to the nucleus
- Describe protein targeting to the mitochondria
- Describe protein targeting to the peroxisomes
- Describe protein targeting to the lysosomes
- Describe protein targeting of transmembrane proteins
- What is receptor-mediated endocytosis?
- Describe clathrin-mediated endocytosis
Protein targeting
Proteins are synthesized on ribosomes in the cytosol. However, proteins are needed in other cellular compartments as well, like the nucleus, ER, lysosomes, peroxisomes, etc. These proteins must be transported from the cytosol and to these targets.
This is done by the help of signal sequences, a short sequence of amino acids on the N-terminus (more common) or C-terminus (less common) of a protein. These sequences are recognized on the membrane of the target organelle, and the polypeptide is transported inside. On the inside, the signal sequence may be cleaved off, but not in all cases, as we shall see.
Because signal sequences are most commonly found on the N-terminus, and because the ribosome synthesize the N-terminus of proteins first, the signal sequences are often the first part of the protein to be translated.
Transport to ER
When a protein is targeted for transport into the ER, a protein called SRP binds to the N-terminus signal sequence, while the protein is still being synthesized. SRP consumes GTP in this process. SRP then “carries” the ribosome, which has now paused the translation, to the ER. When at the ER, the ribosome continues the translation, now “feeding” the translated polypeptide chain directly into the ER. Inside the ER, the signal sequence is removed by a signal peptidase. When translation is finished, the product is now inside ER, and the ribosome dissociates.
Inside the ER, proteins can be further modified, like with disulphide bond formation, or glycosylation. Tunicamycin is an antibiotic that inhibits this glycosylation. Following this, the proteins travel from ER to Golgi in transport vesicles. In Golgi, glycosylated proteins may be further modified. After this, Golgi sorts the proteins, and sends them to their final destinations.
Protein targeting to nucleus
The signal sequence that target a protein for the nucleus is not cleaved off when the protein has entered the nucleus. This is because during cell division, the nuclear envelope is broken down, so all proteins inside are dispersed through the cytoplasm. After cell division, the nuclear envelope is rebuilt, and the proteins must find their way back in. This signal sequence is called the nuclear localization sequence, or NLS. NLS is different from other signal sequences in that it may be found anywhere on the protein, not just on the ends.
A protein called importin is involved in the transport of NLS-tagged proteins into the nucleus.
Protein targeting to mitochondria
Proteins targeted for mitochondria have signal sequences. Unlike proteins targeted for transport into ER, proteins targeted for the mitochondria are synthesized completely in the cytosol before being transported. They are then transported to receptors on the surface of the mitochondria, from where they are transported into the mitochondria, where the signal sequence is removed.
Proteins called Tom20, Tom22, and Tom40 are involved in transport across the outer mitochondrial membrane, while proteins called Tim 22. Tim 23/17, and Tim 44 are involved in transport across the inner mitochondrial membrane.
Protein targeting to peroxisomes
Proteins targeted for transport into peroxisomes contain the signal sequence PTS1 on the C-terminus, or PTS2 on the N-terminus. The PTS1 sequence is one of the few signalling sequences which are located on the C-terminus.
So-called peroxins recognise the PTS signal sequuences and transport them into the peroxisome.
Protein targeting to lysosomes
The signal sequence for proteins targeted to lysosomes is a mannose 6-phosphate residue. On the lysosomal membrane there are mannose 6-phosphate receptors, which bind to and recognize the mannose 6-phosphate residue on the proteins. The protein is then transported into the lysosome.
Protein targeting of transmembrane proteins
Transmembrane proteins, proteins that are embedded in a membrane, reach their target membrane by containing special signal sequences called internal stop-transfer anchor sequences (STA) or internal signal-anchor sequences (SA). These proteins are transported through the membrane they should be embedded in, but when the SA or STA reaches the transporter, the protein is lodged in the membrane.
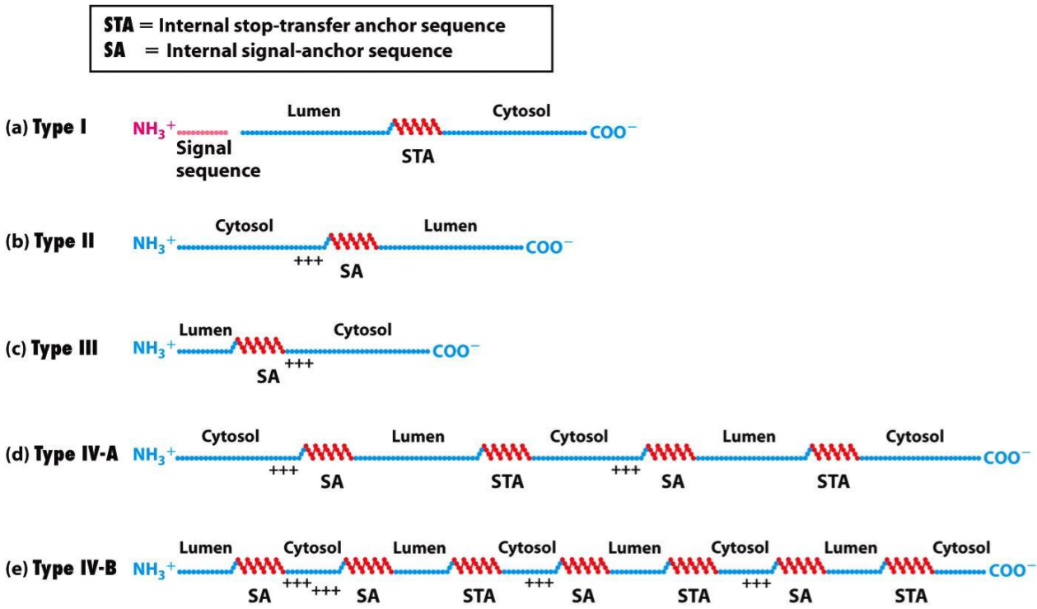
- This figure shows where the different types of transmembrane proteins have their signal sequences.
Type IV transmembrane proteins contain many SA’s and STA’s, so this process is repeated, so that the proteins have 7 parts embedded in the membrane. G-protein coupled receptors are sometimes called 7 transmembrane receptors, or 7TM receptors because of this.
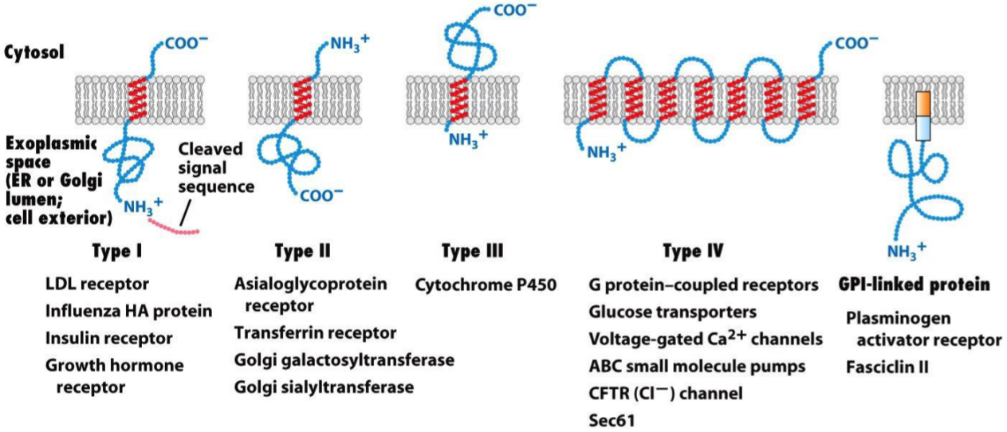
- The different types of transmembrane proteins, with some examples written under.
Receptor-mediated endocytosis
Receptor-mediated endocytosis is a process by which cells can take in extracellular proteins. Cells depend on external proteins to a large degree. For cells to be able to take in for example LDL, transferrin, peptide hormones or circulatory proteins from outside, receptor-mediated endocytosis is needed.
There are three main pathways of receptor-mediated endocytosis. They are the clathrin-dependent endocytosis, caveolin-dependent endocytosis, and clathrin-and caveolin-independent pathways. The clathrin-dependent pathway is the most common.
In clathrin-dependent endocytosis the extracellular protein in question binds to a receptor on the cell surface. The intracellular protein clathrin then binds to the inside of the cell membrane at the area where the extracellular protein has bound to its receptor. Clathrin then forms an invagination of the cell membrane, first forming a pit, and later a vesicle, containing the receptor and the extracellular protein, and internalises it. A protein called dynamin holds the clathrin-coated vesicle together. The extracellular protein dissociated from its receptor inside the vesicle, and the vesicle is subsequently split into two – one vesicle will contain the receptor, which will be moved back to the cell membrane, and the other contains the extracellular protein. The extracellular protein can then be transported to where it is needed.
Summary
- How are proteins targeted for the various organelles?
- By signal sequences, short amino acid sequences on the N-terminus (most common) or C-terminus (rare) of the polypeptide chain
- These signal sequences are recognized by proteins on the target organelle, which transport the protein into the organelle
- Describe protein targeting to ER
- Proteins targeted for the ER have a signal sequence on the N-terminus
- A protein called SRP recognizes this sequence while the polypeptide chain is being translated
- SRP pauses the translation, transports the ribosome and the unfinished polypeptide chain to the ER
- The ribosome then continues translation, directly synthesising the protein into the ER lumen
- Describe protein targeting to the nucleus
- Proteins targeted for the nucleus have a signal sequence called nuclear localization sequence (NLS) inside the polypeptide chain, not on the ends
- Importin recognises the NLS on proteins and transports the protein into the nucleus
- The NLS sequence is not cleaved off, as the protein must be transported back into the nucleus after cell division
- Describe protein targeting to the mitochondria
- Receptors on the mitochondria recognize the target sequence
- Tom20, Tom22 and Tom40 transport the protein across the outer mitochondrial membrane
- Tim22, Tim23/17, and Tim44 transport the protein across the inner mitochondrial membrane
- Describe protein targeting to the peroxisomes
- Proteins targeted for peroxisomes contain the signal sequence PTS1 on the C-terminus, or PTS2 on the N-terminus.
- So-called peroxins recognise the PTS signal sequuences and transport them into the peroxisome
- Describe protein targeting to the lysosomes
- Proteins targeted for lysosomes contain a mannose 6-phosphate residue
- This residue is recognized by mannose 6-phosphate receptors on the lysosomes
- Describe protein targeting of transmembrane proteins
- Transmembrane proteins contain one or more anchor sequences which will function as their anchors in the membrane
- What is receptor-mediated endocytosis?
- Receptor-mediated endocytosis is a pathway by which cells can take in extracellular proteins
- Describe clathrin-mediated endocytosis
- Extracellular proteins bind to their receptor on the cell surface
- Clathrin forms an invagination of the cell membrane at the site, eventually forming a vesicle containing both the receptor and the extracellular protein
- The vesicle splits into two, one containing the receptor, and the other containing the protein