Table of Contents
Page created on March 5, 2018. Last updated on December 18, 2024 at 16:55
Learning objectives
- What is the function of glucokinase?
- How is glucokinase different from other types of hexokinase?
- How are the glycolysis and gluconeogenesis regulated?
- What activates PP2A?
- How does insulin regulate PFK-2/FBPase-2?
- How does glucagon regulate PFK-2/FBPase-2?
- How is pyruvate kinase regulated?
- How does acetyl-CoA regulate gluconeogenesis and glucose breakdown?
Regulation of glucokinase
There are 4 different isotypes of the hexokinase enzyme, which catalyses the phosphorylation of glucose to glucose 6-phosphate. Hexokinase type IV is only found in liver and β cells of the pancreas and is called glucokinase. The first 3 types of hexokinase are found in other tissues and have similar properties, so they’re often referred to as just hexokinase. Glucokinase has different features than hexokinase.
While hexokinase is regulated allosterically, glucokinase is regulated by binding to a regulatory protein. When glucokinase is inhibited it’s bound to the regulatory protein and transported into the nucleus, where it can’t perform its activity. When it is stimulated it is transported back to the cytosol and unbound from the regulatory protein.
The purpose of hexokinase is to sequester glucose inside tissues which need it. As soon as glucose enters the cell through a GLUT transporter, hexokinase will convert it into G6P. Normal cells don’t have glucose 6-phosphatase, so as soon as the glucose is converted into G6P, it will never leave the cell. It will either be used for energy or stored.
The purpose of glucokinase in the liver is to allow the liver to take in large amounts of glucose after a meal to store it as glycogen.
The purpose of glucokinase in β-cells is to allow these cells to “sense” the blood glucose level. When these cells sense that the blood glucose level is high, they’ll respond by producing insulin.
The following table shows the differences between hexokinase and glucokinase.
| Property | Hexokinase | Glucokinase |
|---|---|---|
| Location | Everywhere except β cells, liver | β cells, liver |
| Affinity to the substrate | Higher | Lower |
| Enzyme capacity | Lower | Higher |
| Induced by insulin | No | Yes |
| Inhibited by glucose 6-phosphate | Yes | No |
| Stimulated by glucose | No | Yes |
| Inhibited by fructose 6-phosphate | No | Yes |
| Substrate | All hexoses | Only glucose |
This article describes the differences between glucokinase and hexokinase in more detail, and why they’re different.
The reciprocal regulation of the glycolysis and gluconeogenesis
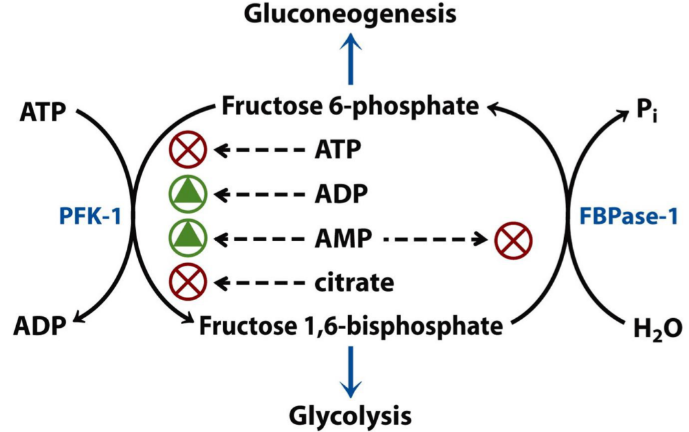
How PFK-1 and FBPase-1 are regulated. Note that the effect of fructose 2,6-bisphosphate is missing from this figure for some reason.
Reciprocal means oppositely regulated; when one is activated, the other is inhibited. This is done to prevent the gluconeogenesis and glycolysis from running at the same time, which would waste energy. PFK-1 is the rate-limiting enzyme of the glycolysis, while FBPase-1 is the rate-limiting enzyme of the gluconeogenesis.
Looking at the figure above, let’s look at citrate as an example of how it works. Citrate is an intermediate in the citric acid cycle (hence the name), and works as a signal to tell enzymes that the cell has plenty of intermediates for energy production, so it doesn’t need more ATP right now. This is why it inhibits PFK-1, so it pushes the equilibrium towards gluconeogenesis instead of glycolysis.
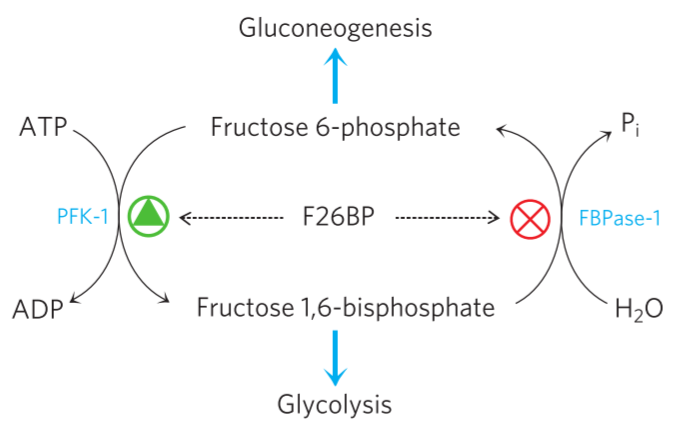
The effect of fructose 2,6-bisphosphate on PFK-1 and FBPase-1.
The most potent regulator of PFK-1 and FBPase-1, and therefore glycolysis and gluconeogenesis, is fructose 2,6-bisphosphate. Insulin and glucagon regulate glycolysis and gluconeogenesis through this molecule.
Fructose 2,6-bisphosphate is produced from fructose 6-phosphate by a large enzyme with 2 catalytic domains. The first domain is called phosphofructokinase-2 (PFK2), which catalyses the formation of fructose 2,6-bisphosphate from fructose 6-phosphate. The second domain is called fructose 2,6-bisphosphatase (FBPase-2). This domain catalyses the reverse reaction, converting fructose 2,6-bisphosphate back to fructose 6-phosphate. These are not two separate proteins but rather two different catalytic domains on the same protein. It’s basically two enzymes stuck together. Let’s call it PFK-2/FBPase-2.
PFK-2/FBPase-2 is covalently regulated; When it is phosphorylated the FBPase-2 domain is active and the PFK-2 domain is inactive. When it is not phosphorylated the PFK-2 domain is active while the FBPase-2 domain is inactive.
When the PFK-2 domain is active, F26BP is formed from F6P. F26BP stimulates the glycolysis and inhibits the gluconeogenesis.
When the FBPase-2 domain is active, F26BP is converted back to F6P. The decreasing levels of F26BP stimulates gluconeogenesis and inhibits glycolysis.
Phosphorylated enzyme -> gluconeogenesis
Dephosphorylated enzyme -> glycolysis
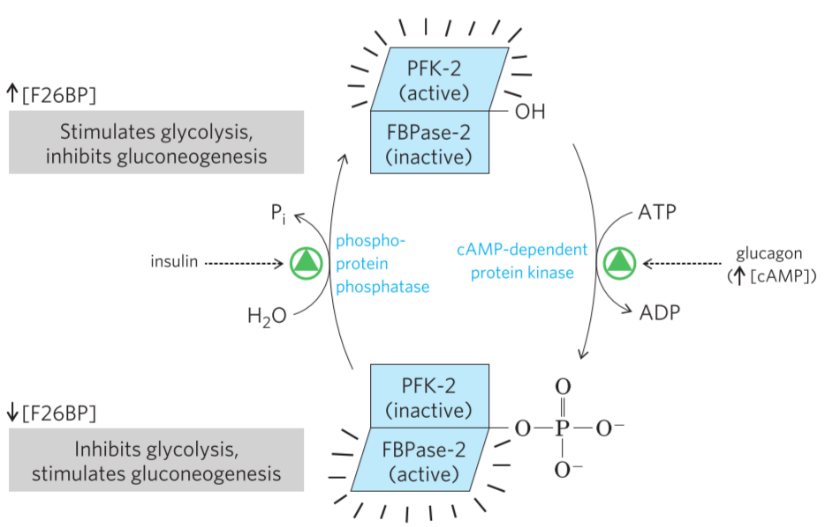
The effect of insulin and glucagon on PFK-2 and FBPase-2. Note that the “phosphoprotein phosphatase” is actually Protein Phospatase 2A, or PP2A. The “cAMP-dependent protein kinase” is actually Protein Kinase A (PKA).
After a meal the blood glucose level is high. There is no need for gluconeogenesis, and cells should instead perform glycolysis to make use of the energy from the meal. Insulin is produced by the pancreas in response to the high blood glucose. Insulin stimulates PP2A, which dephosphorylates PFK-2/FBPase-2. This stimulates the PFK2 domain, increasing levels of F26BP and stimulating glycolysis.
In a fasting state the blood glucose level is low. There is a need for gluconeogenesis. Glucagon is produced by the pancreas in response to the low blood glucose. Glucagon stimulates protein kinase A (PKA), which phosphorylates PFK-2/FBPase-2. This stimulates the FBPase-2 domain, which decreases the levels of F26BP, thereby stimulating gluconeogenesis.
Regulation of pyruvate kinase
Pyruvate kinase is the last enzyme of the glycolysis, which catalyses PEP -> pyruvate. From here pyruvate has many fates:
- It can be converted to lactate as part of anaerobic glycolysis
- It can be converted to acetyl-CoA
- Which can enter the TCA cycle, be used for ketone body synthesis, or for fatty acid synthesis
- It can be converted to oxaloacetate to be used in gluconeogenesis
- It can be converted to alanine as part of the Cahill cycle (not so important)
In all tissues pyruvate kinase is inhibited by ATP, acetyl-CoA and long-chain fatty acids. This makes sense, as all three of these are present when the body has enough energy, so pyruvate kinase shouldn’t be needed.
Pyruvate kinase is stimulated by fructose 1,6-bisphosphate, as a feed-forward stimulation of the glycolysis. After all, there’s no point in beginning the glycolysis without finishing it? PEP alone is of no use to anyone.
L-type pyruvate kinase, which is found in the liver, is also regulated by hormones. Glucagon inactivates pyruvate kinase via PKA to stop glycolysis. Insulin activates it via a protein phosphatase. Either PP1 or PP2A, the lecture doesn’t specify.
Role of Acetyl-CoA in regulation
Acetyl-CoA inhibits pyruvate dehydrogenase complex as a negative feedback mechanism. It also activates pyruvate carboxylase, which is used to push the equilibrium between glycolysis/gluconeogenesis slightly toward gluconeogenesis.
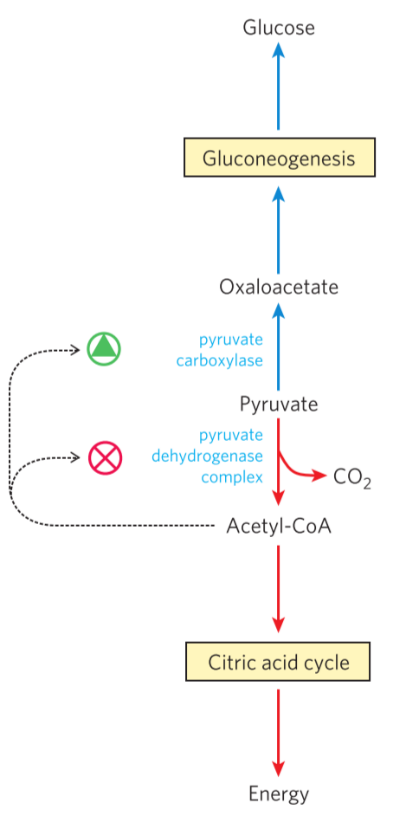
How acetyl-CoA regulates things.
Summary
- What is the function of glucokinase?
- In the liver glucokinase’s large capacity allows it to take in large amounts of glucose to be stored as glycogen after a meal
- In β-cells glucokinase functions as a glucose sensor, allowing the cell to monitor the blood glucose level
- How is glucokinase different from other types of hexokinase?
- It is not inhibited by its product G6P
- It has higher capacity
- It has lower affinity
- It is regulated by a regulatory protein rather than allosterically
- How are the glycolysis and gluconeogenesis regulated?
- Citrate and ATP stimulates gluconeogenesis and inhibits glycolysis
- ADP and AMP stimulates glycolysis and inhibits gluconeogenesis
- F26BP stimulates glycolysis
- What activates PP2A?
- Xylulose 5-phosphate
- How does insulin regulate PFK-2/FBPase-2?
- Insulin activates PP2A, which dephosphorylates PFK-2/FBPase-2, stimulating glycolysis and inhibiting gluconeogenesis
- How does glucagon regulate PFK-2/FBPase-2?
- Glucagon activates PKA, which phosphorylates PFK-2/FBPase-2, stimulating gluconeogenesis and inhibiting glycolysis
- How is pyruvate kinase regulated?
- Stimulated by fructose 1,6-bisphosphate
- Inhibited by ATP, acetyl-CoA, and long-chained fatty acids
- In the liver, pyruvate kinase is stimulated by insulin and inhibited by glucagon
- How does acetyl-CoA regulate gluconeogenesis and glucose breakdown?
- It inhibits pyruvate dehydrogenase complex
- It stimulates pyruvate carboxylase
i have one more question about the regulation of carbohydrate mechanism ! So in case of low glucose lv , glucagon is produced so further it will activate PKA by cAMP so they will activates process that produce atp and itactivates process that use atp which means they will activate glycolysis and inactivate gluconeogenesis right? but here is the thing that i can’t understand . In contrast insuline mechanism they do opposite way, Glucose lv increased so isk is activated and PI3K >PDK>PKB finally they all inactivate gluconeogenesis and activate glycolysis to reduce glucose lv but they are opposite mechanism. Now i am inside of miro. haha.,
You seem to be misunderstanding one point.
The goal of glucagon is to increase the level of glucose in the blood. As such, glucagon inactivates glycolysis (which consumes glucose) and stimulates gluconeogenesis (which forms glucose), not the other way around.
As you said, the goal of insulin is the opposite, to decrease the level of glucose in the blood. As such, insulin inhibits gluconeogenesis and stimulates glycolysis.
Also, next time you leave a comment, don’t include an e-mail, as it makes your comment go directly to trash. It was a coincidence that I saw this one.
Thank you! do you know what are the substrates of glycogen synthase? is it only glucose or glucose> g6p>g1p>udp-glucose ?
The glycogen synthase enzyme catalyses only one reaction:
Glycogen(n) + UDP-Glc -> Glycogen(n+1) + UDP
In other words, it takes a glycogen molecule (of length “n”) and adds the glucose part of a UDP-glucose molecule to it. thereby making it length “n+1”.
As such, the only substrate of the enzyme is UDP-glucose.