Table of Contents
Page created on May 1, 2018. Last updated on December 18, 2024 at 16:56
There are many ways for a cell to die, both programmed (intentionally) and accidental (unintentionally). The body may want to kill off cells in certain processes, for example in T-cell selection in the thymus, affinity maturation of B-cells in the secondary lymphoid organs or in the case of sick or infected cells. The three main ways of programmed cell death are apoptosis, autophagy and more recently discovered, necroptosis (not to be confused with necrosis). Apoptosis is the “normal” PCD with the other two being “backup” mechanisms in case apoptosis is unsuccessful or impossible.
Apoptosis
Apoptosis is dependent on a group of proteins called caspases. Caspases are cysteine-containing enzymes that cleave proteins at aspartate residues. They activate another enzyme called caspase-dependent DNase (CAD), which cleaves DNA to kill the cell. There are two main pathways of apoptosis, the intrinsic and the extrinsic pathway.
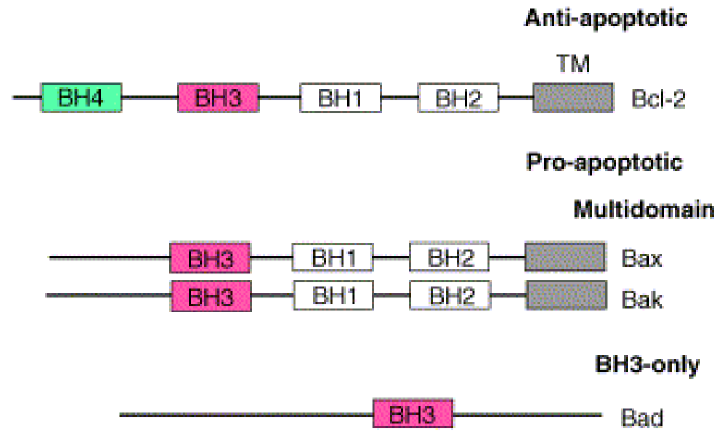
Many proteins are involved. Bcl-2 is an anti-apoptotic protein, while BAD, BAX and BAK are pro-apoptotic. Bcl-2 contains 5 segments. BH1, BH2, BH3, BH4 and a TM segment. The BH4 segment is the segment that makes Bcl-2 anti-apoptotic. BAK and BAX contain the same segments except BH4, which make them pro-apoptotic. BAD contains only BH3, and is therefore also pro-apoptotic as it lacks BH4.
The intrinsic pathway is largely dependent on the mitochondria. Certain stimuli such as DNA damage, ER stress, hypoxia or metabolic stress. These stimuli activate BAD. BAD then activates BAX and BAK which form a pore in the outer mitochondrial membrane called MAC. This increases the permeability of the outer mitochondrial membrane, a process called mitochondrial outer membrane permeabilization or MOMP, which allows molecules from inside the mitochondria will migrate out into the cytoplasm. In particular, cytochrome C, DIABLO (or SMAC), AIF and EndoG are released.
DIABLO is a protein that inhibits a protein that inhibit caspases called XIAP, thereby activating caspase-3 and caspase-7, allowing them to induce apoptosis. Cytochrome C forms a complex with a cytoplasmic protein called APAF-1 to form a complex called the apoptosome. The apoptosome activates caspase-9, which again activates caspase-3 and caspase-7. AIF and EndoG both degrade DNA without the use of caspases.
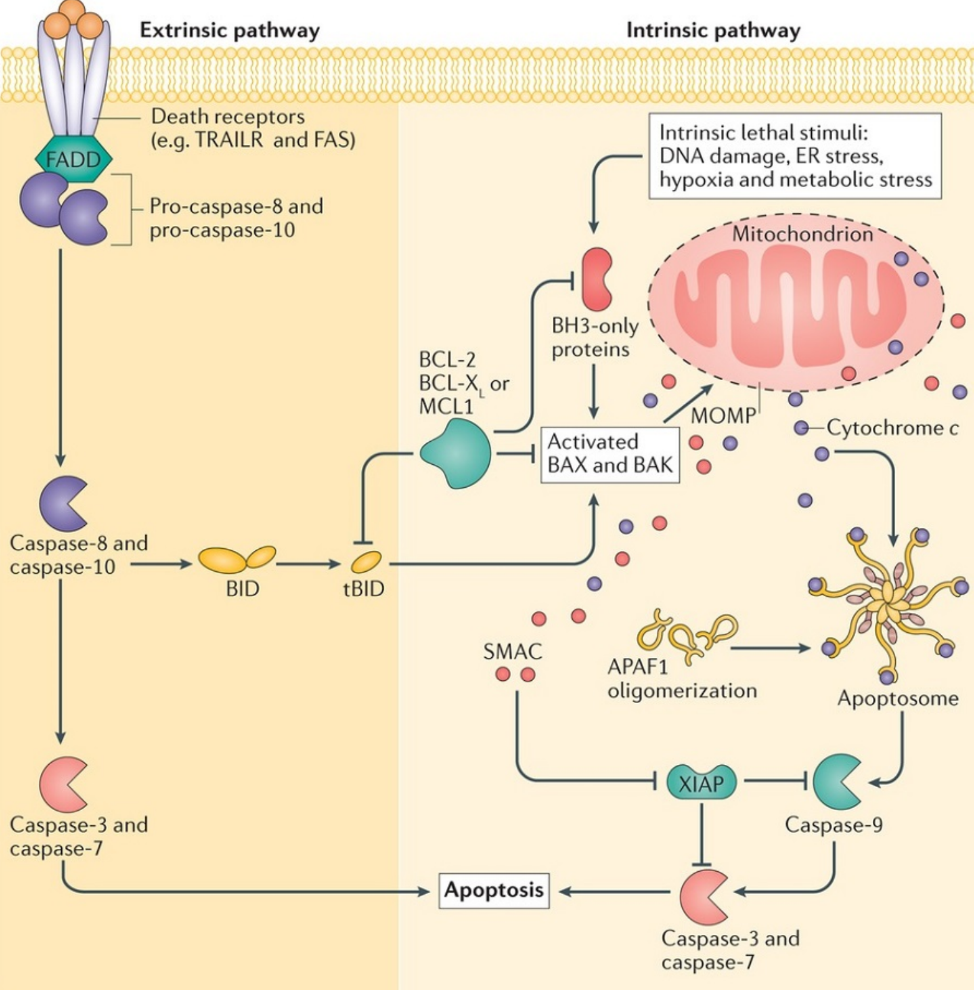
The extrinsic pathway is activated by the binding of certain ligands to a special receptor on the cell surface. These ligands are often produced by the immune system to kill infected or cancerous cells. These ligands are TNF-α which binds to TNFR1, or FAS-L which binds to FAS receptor. Both receptors bind pro-caspase 8 to convert it to caspase 8, which activates BAK and BAX to activate the intrinsic pathway. Caspase-8 also directly activates caspase-3 and caspase-7.
Apoptosis ends with all cellular components being packaged in vesicles called apoptotic bodies and released into the extracellular space. The apoptotic bodies will then be phagocytosed and degraded by macrophages without inducing inflammation.
Autophagy
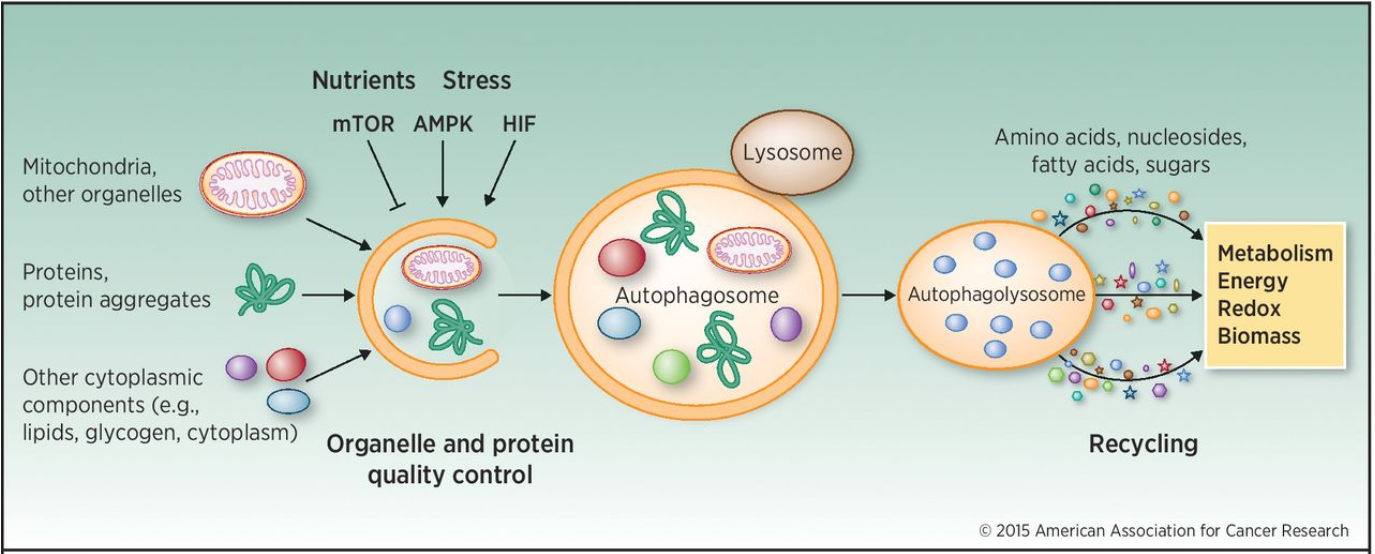
The name means “eating itself”, which is mostly what’s happening here. Autophagy is not necessarily a cell death process as it’s also used in removing unused cellular components in homeostasis. It involves packaging cellular components like mitochondria, proteins and other organelles inside a double-layered membrane, forming an autophagosome. This autophagosome will then fuse with a lysosome to become an autophagolysosome, where proteases called cathepsins will degrade its contents to be used for recycling.
Necroptosis
Necroptosis is a form of programmed necrosis. It’s caspase-independent and is therefore suitable for the programmed cell death of cells infected with viruses that produce caspase inhibitors. Necroptosis, like necrosis, leads to the cellular components being leaked into the extracellular space. Like the extrinsic pathway of apoptosis, it is activated by binding of TNF-α to TNFR1. This activates a kinase called RIPK1.
RIPK1 activates NK-κB which initiates a cascade which ends with the formation of a complex of RIPK1 and RIPK3 called the necrosome. The necrosome then forms a pore in the cell membrane, which causes cell lysis and death.
Necrosis
If apoptosis is the “clean” way to kill a cell, then necrosis is the opposite. Necrosis is a pathological cell death that occurs when a cell is exposed to serious physical or chemical insult or ATP level collapse. Some factors than can induce necrosis are viruses, hypothermia, hypoxia, ischemia or metabolic problems. The main problem with necrosis is that it induces a significant inflammatory response.
The key mechanism in necrosis is that there is ATP depletion. This can be because hypoxia or ischemia limits how much nutrients the cell receives, or due to toxins inhibiting ATP production. Because the cell doesn’t have any ATP left, it doesn’t have energy to package cell components into vesicles like in apoptosis. It doesn’t have energy to maintain ion balance either, which causes osmotic swelling of the cell. This will eventually cause the cell membrane to rupture, which releases the intracellular components into the extracellular space. When these components are later phagocytosed by macrophages, the macrophages will produce inflammation.
Special types of cell death
Pyroptosis is a highly inflammatory form of programmed cell death that occurs in immune cells when they recognize that they’ve been infected. It causes activation of caspase-1 which forms pores in the cell and causes lysis and death.
Paraptosis is activated by IGF and not caspase-dependent. It causes inflammation.
A mitotic catastrophe occurs when stress disrupts one of the checkpoints of the cell duplication cycle. This causes the cell to continue with mitosis even though something is wrong, which causes abnormal daughter cells.
Ferroptosis is caused by a lack of glutathione peroxidase (GXP4). When there is a lack or deficiency of this enzyme, lipid reactive oxygen species will form and cause cell death.
Anoikis is a type of PCD that occurs when a cell detaches from the ECM it belongs to. This process is defective in metastatic tumors, which means that the cancer cells don’t kill themselves when they migrate someplace they shouldn’t be.