Table of Contents
Page created on October 20, 2019. Last updated on December 18, 2024 at 16:55
[H+] means the concentration of H+ ions. The brackets mean “concentration”.
pH
The pH of a solution depends on the solution’s concentration of hydrogen ions (H+). The higher the concentration of H+ ions, the lower the pH and vice versa.
The pH of a solution is determined by the following formula: pH = -log(concentration of H+) or pH = -log([H+]).
If we know the pH we can calculate the H+ concentration by this formula: [H+] = 10-pH
pH is a logarithmic scale, meaning that a tenfold increase in H+ concentration decreases the pH by one. A decrease in pH by 4 units equals an increase in H+ concentration 10 000-fold.
Blood pH
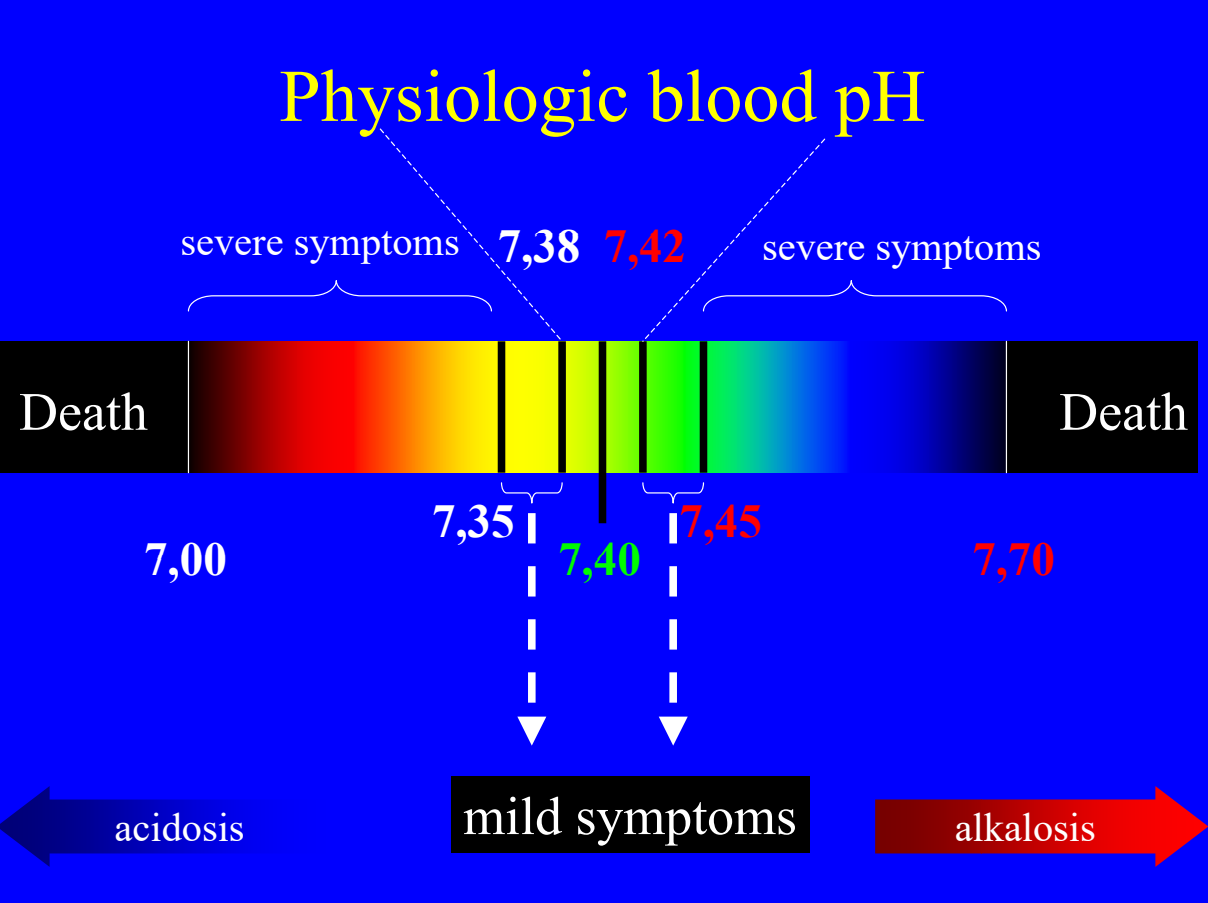
The H+ concentration of the blood is precisely regulated. Even small changes in blood pH can be fatal. The H+ concentration is tightly regulated so that the pH of the arterial blood is between 7,38 and 7,40 at all times. A pH of 7,35 – 7,38 or 7,42 – 7,45 gives mild symptoms. A pH of 7,00 – 7,35 or 7,45 – 7,70 gives severe symptoms. A pH of less than 7,00 or more than 7,70 is deadly.
Credits to physiology department
The word acidosis describes a person whose arterial blood pH is lower than 7,38, while the word alkalosis describes a person whose pH is above 7,42.
The reason the pH is so tightly regulated is because the H+ concentration influences the activity of enzymes. Thus, an abnormal pH will cause abnormal function of enzymes, which are essential for the body to function properly.
The concentration of H+ in arterial blood is 40 nMol/L. The concentration of H+ in venous blood is slightly higher, 44 nMol/L, which is why venous blood is slightly more acidic than arterial blood.
The body constantly produces acids, so it must constantly get rid of acids too. There are three major mechanisms involved in pH regulation:
- Buffer systems
- The lungs
- The kidneys
Important normal values:
- Arterial blood pH: 7,38 – 7,42
- Venous blood pH: 7,35
- Arterial pCO2: 40 mmHg
- Venous pCO2: 46 mmHg
- Arterial [HCO3]: 24 mMol/L
Buffer systems
A buffer is a chemical system which resists changes to pH. It’s comprised of an acid and a base in approximately equal amount. Buffers don’t remove H+ from the body but they “tie them up” by binding them to other molecules until the excess H+ can be removed by the lungs or the kidneys. Buffers act immediately, as soon as a change in pH occurs.
The blood contains many buffers:
- In the plasma
- Bicarbonate buffer – 35%
- Proteins – 7%
- Phosphate – 2%
- In red blood cells
- Bicarbonate buffer – 18%
- Haemoglobin buffer – 35%
The percentages show how much each buffer contributes to regulating the pH.
Sources of acid:
Acid is produced in the body as part of the metabolism. When cells break down sugar (glucose), fatty acids or proteins into energy they also produce large amounts of CO2 and some amounts of metabolic acids.
About 80 millimoles of H+ is produced each day by the metabolism. That’s more than a million times more H+ which is present in the blood. It’s obvious that the body produces lots of acid which must be buffered and removed, otherwise severe acidosis would develop very quickly.
Bicarbonate buffer system:
The bicarbonate buffer system is arguably the most important buffer in the body. It consists of H2CO3, a weak acid, and HCO3–, a weak base. They exist in a chemical equilibrium, like this:
H2CO3 <-> HCO3– + H+
H2CO3 is formed in the body from CO2 and H2O in the following chemical reaction:
CO2 + H2O <-> H2CO3
This chemical reaction is catalysed by the enzyme carbonic anhydrase. The bicarbonate buffer system would not work without this enzyme.
If we put the two above equations together, we can write the bicarbonate buffer system like this:
CO2 + H2O <-> HCO3– + H+
By putting the bicarbonate buffer system in the Henderson-Hasselbalch equation, we get the following formula:
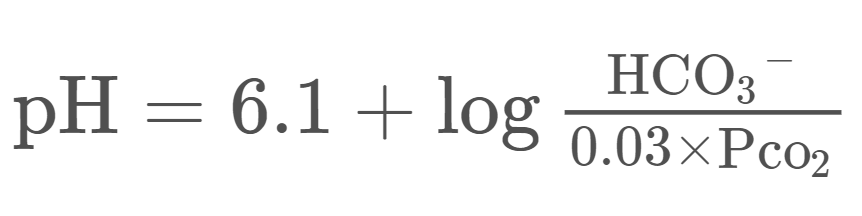
For complicated reasons we write the CO2 as 0,03 x pCO2 when working with the blood. Because CO2 is a gas, we measure its partial pressure in the blood, rather than its concentration. The partial pressure of CO2 is written as pCO2. Because pCO2 is a measure of pressure its unit is mmHg (millimetres of mercury).
From this equation we can understand that:
- The pH increases if the concentration of HCO3– increases
- The pH increases if the partial pressure of CO2 (pCO2) decreases
- The pH decreases if the concentration of HCO3– decreases
- The pH decreases if the partial pressure of CO2 (pCO2) increases
The normal value of the concentration of HCO3– is 24 mMol/L and the normal value of the pCO2 is 40 mmHg. You can plug these values into the above concentration and see that pH will be 7,4.
Other buffer systems:
Haemoglobin is the protein which is most abundant in the blood. Almost any protein can act as a buffer, including haemoglobin. H+ can bind to the proteins. H+ and proteins exist in an equilibrium like this:
H+ + protein– <-> H-protein
Histidine is an amino acid. It is the histidine residues of haemoglobin which are the major proton acceptors. Each histidine residue can bind one H+, and one molecule of haemoglobin contains 38 histidine residues. This means that each molecule of haemoglobin can bind 38 H+ ions.
Removal of H+ by the lungs
The lungs don’t remove H+ directly, but indirectly. We constantly breathe out CO2. This decreases pH as we saw from the equation above. However, the body produces small amounts of other acids as well, and the lungs cannot contribute to the removal of these acids.
When there is acidosis, i.e. the concentration of H+ is too high, the brain stem will stimulate breathing. When breathing increases more CO2 is removed from the body, which increases the pH back to normal.
However, when there is alkalosis, the lungs can’t contribute much to restoring the normal pH. The brainstem might decrease breathing a bit, but it obviously can’t change it so much that the body will become hypoxic. As such, the lungs are much more important in compensation of acidosis than of alkalosis.
The respiratory regulation of occurs very quickly but not as quick as the buffers.
Removal of H+ by the kidneys
The kidneys control acid-base balance by excreting either acidic or basic urine. Excreting acidic urine increases the blood pH while excreting basic urine decreases the blood pH. Unlike the lungs the kidneys can contribute to the removal of all acids the body produces.
You’ll learn about how the kidney works later, but the gist of it is that the kidney filters out molecules from the blood, including H+ and HCO3–.
When there is acidosis the kidney will excrete more H+ and excrete less HCO3–. When there is alkalosis the kidney will excrete less H+ and more HCO3–.
The kidneys need some time to start compensating for pH changes, approximately 1 – 2 days.
Acidosis and alkalosis
The body is better equipped to deal with acidosis than with alkalosis. This is due to the fact that the lungs cannot participate in the management of alkalosis.
We can divide both acidosis and alkalosis according to their cause. We call them either respiratory or metabolic, according to whether the cause of the pH disturbance is respiratory or metabolic in origin.
- Acidosis
- Metabolic acidosis
- Respiratory acidosis
- Alkalosis
- Metabolic alkalosis
- Respiratory alkalosis
Metabolic acidosis:
Metabolic acidosis occurs when the body produces or ingests too much H+ or loses too much HCO3–. Here are some possible causes:
- Increased production of H+
- Lactic acidosis
- Ketoacidosis
- Heavy exercise
- Increased ingestion of acid
- Consumption of methanol or other toxic compounds
- Increased loss of HCO3–
- Diarrhoea – diarrhoea contains much HCO3–
Lactic acidosis is what we call the metabolic acidosis that’s caused by increased production of lactic acid. Lactic acid is a by-product of anaerobic metabolism, metabolism which occurs in the absence of enough O2. Anaerobic metabolism occurs during exercise and if the body doesn’t have enough oxygen (hypoxia).
Ketoacidosis is what we call the metabolic acidosis that’s caused by production of ketone bodies. Ketone bodies are alternative energy sources for the body, produced by the liver during starvation and in diabetes. Ketone bodies are also acids and will therefore cause acidosis.
The body will compensate for metabolic acidosis by stimulating respiration. This is called hyperventilation. If the acidosis is severe enough the breathing may become deep and laboured. This breathing pattern is called Kussmaul breathing and is characteristic for severe metabolic acidosis, especially diabetic ketoacidosis.
The body will also compensate by decreasing renal HCO3– excretion.
Respiratory acidosis:
Respiratory acidosis occurs when a person cannot breathe enough to get rid of enough CO2, causing CO2 to accumulate. This is called hypoventilation and occurs when something prevents the person from breathing normally. It can be caused by:
- Chronic obstructive pulmonary disease (COPD) – the lung disease you get from smoking
- Muscle weakness involving the diaphragm
- Other lung diseases
The body will compensate by decreasing renal HCO3– excretion.
Metabolic alkalosis:
Metabolic alkalosis occurs when the body produces or ingests too much HCO3– or loses too much H+. Causes:
- Vomiting – as you lose stomach acid
- Excessive intake of HCO3–
The body will compensate mainly by increasing renal HCO3– excretion. It will also slightly inhibit respiration, which will retain more CO2.
Respiratory alkalosis:
Respiratory alkalosis occurs when a person hyperventilates, so the CO2 level in the body decreases. Causes:
- Voluntary hyperventilation
- High altitude
In high altitude the air contains less oxygen. To compensate the brain stem will stimulate breathing, which allows the body to get the oxygen it needs. However, this can also wash out too much CO2, causing respiratory alkalosis.
The body will compensate by increasing renal HCO3 excretion.
Calcium and blood pH
The blood pH directly affects the calcium level in the blood. When the pH decreases the calcium, level increases and vice versa.
Calcium in the blood exists in two fractions:
- As a free calcium ion (Ca2+) – the “free” fraction
- Calcium bound to proteins in the blood – the “protein-bound” fraction
The total amount of calcium in the blood is 2,4 mMol/L. 50% of this (1,2 mMol/L) are free calcium ions and 50% of this is calcium which is bound to proteins. However, when we talk about hypocalcaemia or hypercalcaemia, we only refer to the free fraction.
Only the calcium which is in free ion form is biologically active; the calcium which is bound to proteins doesn’t affect anything. In other words, it’s the free calcium ion fraction which decides calcium’s “activity” in the body. When the free calcium ion fraction increases, problems can occur, but when the protein-bound fraction increases nothing happens.
When the pH decreases the H+ concentration increases. When the H+ concentration increases the proteins in the blood will bind more H+, which causes them to bind less Ca2+. This causes more calcium in the blood to be in the free fraction and less to be in the protein-bound fraction. This causes hypercalcaemia. Likewise, when the pH increases there will be hypocalcaemia.
This is important because hypercalcaemia causes muscle weakness and hypocalcaemia causes uncontrolled muscle contractions, called tetany, which can be deadly if the muscles of the larynx are affected. Both hypercalcaemia and hypocalcaemia can cause arrhythmias in the heart.
Summary:
- When there is acidosis there will also be hypercalcaemia
- When there is alkalosis there will also be hypocalcaemia