Table of Contents
Page created on November 3, 2020. Last updated on December 18, 2024 at 16:55
Learning objectives
- What does excitation-contraction coupling mean?
- What do we call the cell membrane, cytoplasm, and endoplasmic reticulum of a muscle cell?
- Describe the composition of skeletal muscle
- Which proteins comprise the thin and thick filaments?
- What is the function of troponin and tropomyosin?
- What is the functional unit of striated muscle?
- What are the thin filaments anchored to?
- What are T-tubules?
- Which two proteins in the T-tubules are essential for excitation-contraction coupling, and what are their functions?
- Describe the mechanism of excitation of skeletal muscle
- Describe the cross-bridge cycle
- Describe the termination of contraction
General principles of muscles
There are three types of muscle tissue. Striated muscle, which includes skeletal and cardiac muscle, and smooth muscle.
Excitation-contraction coupling
Depolarization of the motor end plate initiates a series of events which eventually cause Ca2+ to be released from the sarcoplasmic reticulum of the muscle cell. Skeletal muscle contraction occurs when the level of Ca2+ in the cytoplasm increases.
The depolarization of the motor end plate initiates muscle contraction. For that reason, we say that excitation and contraction are coupled, a phenomenon aptly called excitation-contraction coupling, or electromechanical coupling. This occurs not only in skeletal muscle but in cardiac muscle as well.
General structure of muscle cells
Muscle cells have structures similar to other cells, but because they’re different in some ways they have special names. The cell membrane of a muscle cell is called the sarcolemma, its cytoplasm is called sarcoplasm, and its endoplasmic reticulum is called sarcoplasmic reticulum (SR).
The parts of muscle cells responsible for the contraction itself are the so-called thick and thin filaments. These filaments lie parallelly to each other, and contraction occurs when they slide across each other.
The thick filaments are approx. 15 nm thick and are comprised of a protein called myosin. Each molecule of myosin is shaped like a golf-club, with a long thin part and a roundish head. These heads stick out of the thick filaments and can bind to actin.
The thin filaments are approx. 7 nm thick and are comprised mostly of a protein called actin, but it also contains the proteins troponin, tropomyosin and nebulin. The function of tropomyosin is to prevent myosin from binding to actin during rest.
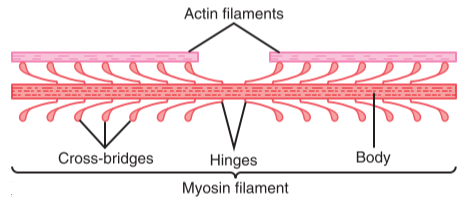
This figure shows the myosin heads and how they stick out from the thick filament. Here the terms actin and myosin filaments are used instead of thin and thick filaments, respectively. From Guyton’s Textbook of Medical Physiology
Interactions between the actin and myosin proteins are what drive the contractions themselves, causing the thick and thin filaments to slide on each other. Unfortunately, there are other proteins involved, and the exact mechanism of contraction differs between muscle types. The general mechanism is similar across all three types, though.
Structure and contraction of skeletal muscle
Structure of skeletal muscle
Each skeletal muscle consists of individual muscle fibres. Each fibre is a cell with many cell nuclei, and each fibre behaves as a single unit. Each muscle fibre contains many chains of myofibrils.
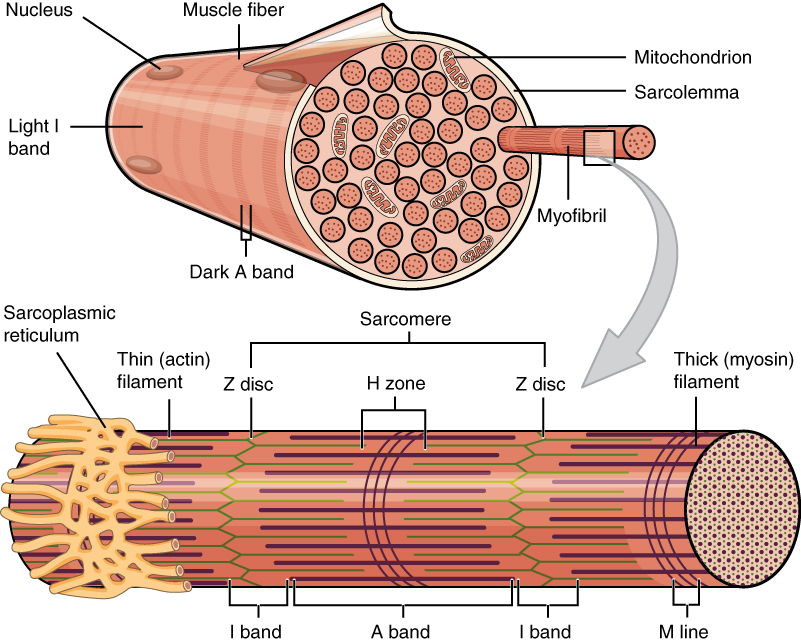
This image shows one muscle fibre in cross-section. In this section it’s easy to see the individual myofibrils which make up the muscle fibre. Thin filament in green and thick filament in reddish-brown something. From https://en.wikipedia.org/wiki/Muscle_contraction
The thick and thin filaments are organized in so-called sarcomeres, found in the myofibrils, as seen above. The sarcomere is the functional unit of striated muscle. The sarcomeres are separated from one another by the so-called Z-lines. The thin filaments are anchored on these Z-lines.
The I-band represents the part of the sarcomere where there are only thin filaments. The A-band represents the whole length of the thick filament. As seen on the image above, the A-band contains both thick and thin filaments.
During contraction, both the thin and thick filaments themselves remain the same length. However, they will slide on each other, which brings the Z-discs closer together and decreases the size of the I-band, as well as the length of the sarcomere.
The sarcolemma of skeletal and cardiac muscle has deep invaginations which penetrate into the muscle fibres. These are called T-tubules, short for transverse tubules. The T-tubules open into the sarcoplasmic reticulum at the so-called terminal cisternae.
The action potential which causes muscle contraction propagates along the sarcolemma and the T-tubules. The network of T-tubules allows the action potential to propagate through the muscle cell much faster.
The T-tubules contain a protein which is important for excitation-contraction coupling, called the dihydropyridine receptor (DHPR). The nearby sarcoplasmic reticulum contains a different protein called the ryanodine receptor, which is a calcium channel. The DHPR is voltage-sensitive, meaning that it is activated when depolarization occurs. The activated DHPR then opens the ryanodine receptor.
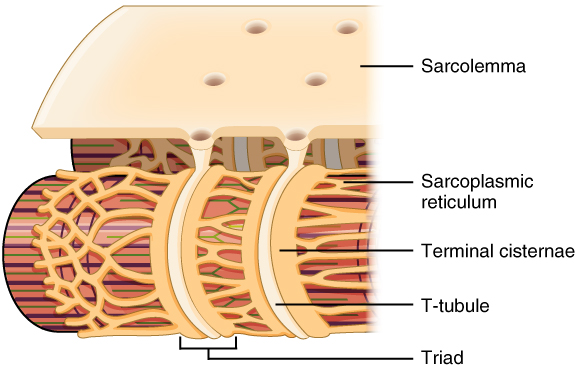
This image shows the T-tubules as invaginations of the sarcolemma, as well as the terminal cisternae through which the T-tubules communicate with the sarcoplasmic reticulum. From https://en.wikipedia.org/wiki/T-tubule
Mechanism of excitation
When the alpha motoneuron creates an action potential, it will reach the neuromuscular junction. The neuromuscular junction will form a new action potential on the motor endplate. This action potential will spread along the sarcolemma of the skeletal muscle cell and into the T-tubules.
The action potential will activate the dihydropyridine receptors in the T-tubules, which will open the ryanodine receptors, allowing Ca2+ to flow out from the sarcoplasmic reticulum and into the sarcoplasm.
Mechanism of contraction
During rest, when there is no contraction, the protein tropomyosin prevents actin and myosin from interacting with each other, thereby preventing contraction.
Before contraction, the myosin heads hydrolyse one ATP into ADP and inorganic phosphate. This hydrolysis puts the head in a high-energy state: we say that the head is now “cocked”.
After excitation, the intracellular level of Ca2+ is increased. Ca2+ binds to the protein troponin on the thin filaments. This moves tropomyosin out of the way. The so-called cross-bridge cycle begins.
- Now that there is nothing to prevent acting and myosin from interacting, the myosin head will immediately bind to actin
- This binding causes a conformational change in the myosin head, which causes the head to move toward the M-line, pulling the thin filament with it. This is called the power stroke. The energy for this comes from the ATP which was hydrolysed before the cycle started. After the power stroke the head is now “uncocked”
- After the power stroke, another molecule of ATP binds to myosin. This causes the myosin head to be released from actin
- The ATP molecule is hydrolysed into ADP and Pi, resetting the head into the “cocked” position
- The myosin head binds to actin in the thin filament again, but this time at a different site
This is similar to the cycle which occurs during rowing:
- The oars are pulled, dragging the boat forward (the power stroke)
- The oars are lifted from the water
- The oars are moved forward (the hydrolysis of ATP)
- The oars are immersed into the water
The cross-bridge cycle repeats as long as the intracellular Ca2+ level remains increased and there is ATP available. Approx. 10 – 100 cycles occur per second.
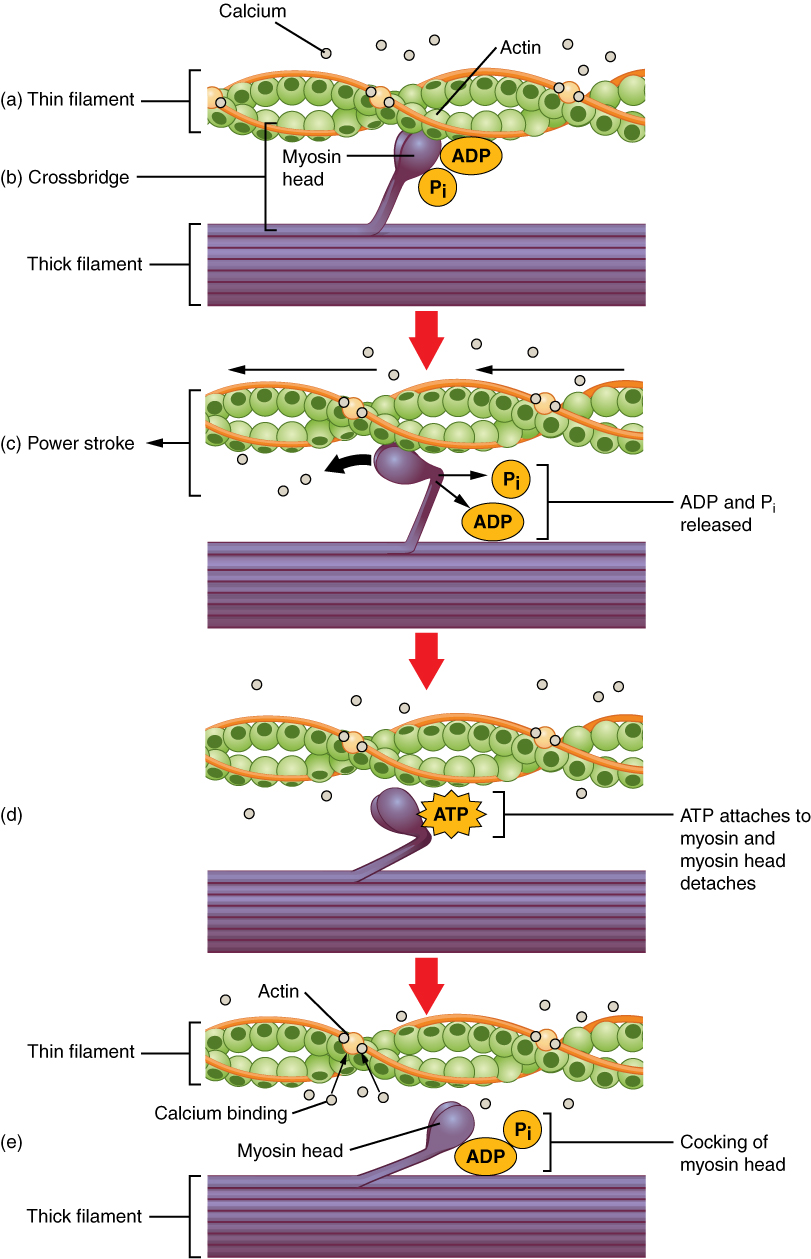
The cross-bridge cycle. From https://en.wikipedia.org/wiki/Muscle_contraction
Rigor mortis is a phenomenon where, soon after death, the muscles of the person become rigid and stiff. This can be explained by the mechanism of muscle contraction. After death, no more ATP is produced. ATP is necessary to make the myosin head dissociate from actin, so when no ATP is available, myosin and actin will remain attached, causing the sarcomere to remain at a fixed length.
Termination of contraction
Muscle contraction usually stops when the alpha motoneuron stops sending action potentials. This allows the sarcolemma and T-tubules to repolarize, which closes the ryanodine receptors. Ca2+ ions are pumped back into the sarcoplasmic reticulum. The decrease in intracellular Ca2+ causes tropomyosin to again prevent actin and myosin from interacting.
If the muscle runs out of ATP due to fatigue, contraction also stops.