Table of Contents
Page created on November 23, 2018. Last updated on December 18, 2024 at 16:56
Ras
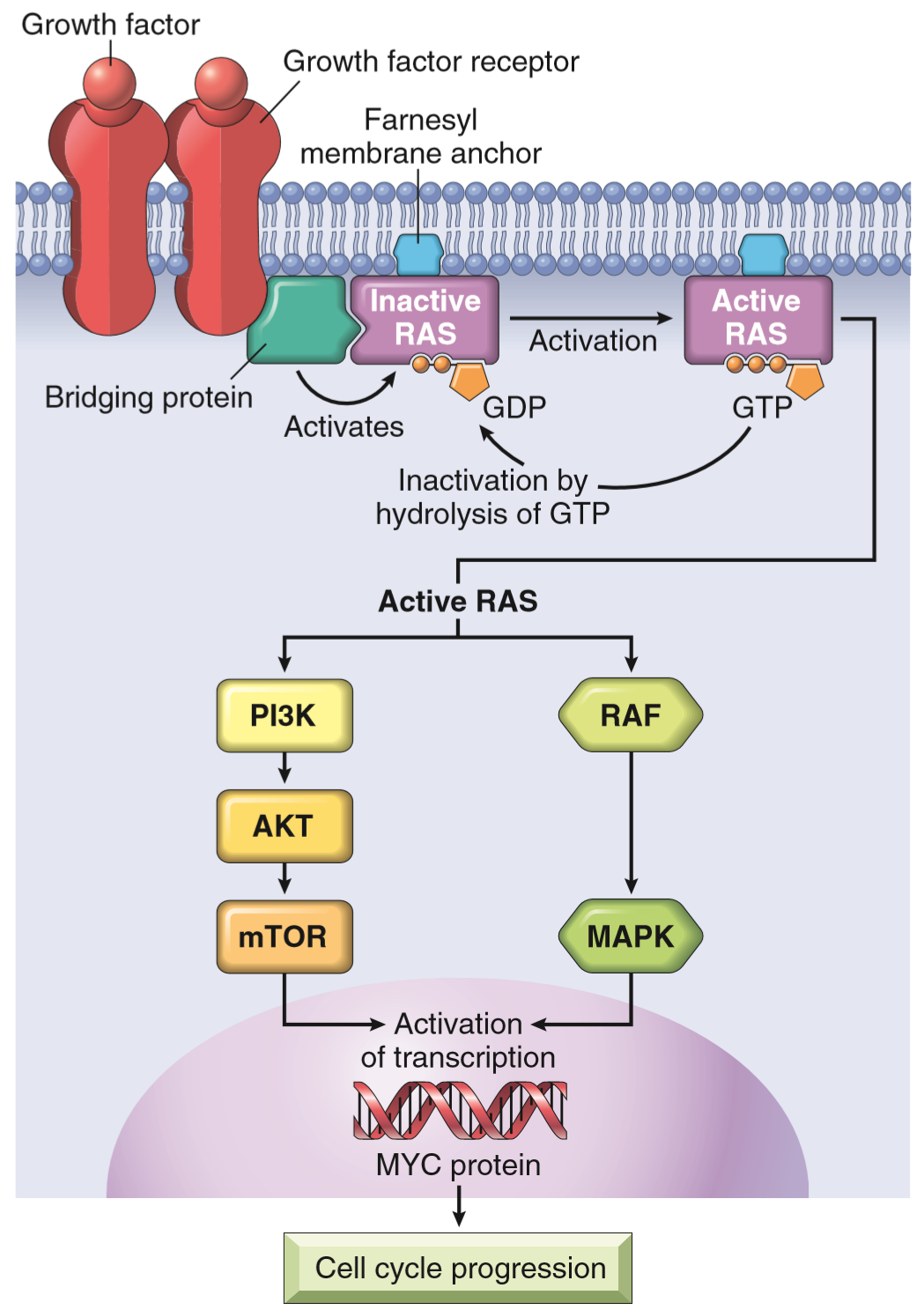
The proto-oncogene Ras is a family of a type of signal transduction proteins called small GTPases. They’re associated with G-protein coupled receptors, another receptor type other than tyrosine kinase receptor. The order of signal transduction for Ras proteins go like this:
- G-protein coupled receptor binds its ligand, which activates the receptor
- The activated receptor causes the Ras protein to bind a GTP, which activates Ras
- Ras then activates other proteins further down the signal transduction pathway
- After this will Ras hydrolyse its GTP to a GDP, which inactivates Ras
- Ras is now inactivated and can become activated the next time the receptor binds its ligand
The problem here occurs when Ras is mutated, so they lose the ability to perform step 4, and therefore stays activated permanently. This will, like was the case for tyrosine receptor kinases, trick the cell to believe that it’s constantly getting stimulated by growth factors, causing it to proliferate.
The point mutation affects one of two parts of the Ras protein, either:
- The GTP binding pocket or
- The enzymatic region responsible for GTP hydrolysis
Three types of Ras exist in humans, HRAS, KRAS and NRAS. In as much as 15-20% of all human cancers is one of the Ras genes point mutated, making it the most common genetic mutation in cancer. In some cancers, it’s incredibly frequent, like:
| Frequency | Cancer type | Affected gene |
| 90% | Pancreas adenocarcinoma | KRAS |
| 90% | Cholangiocellular carcinoma | KRAS |
| 50% | Colorectal cancer | KRAS |
| 50% | Endometrial carcinoma | KRAS |
| 50% | Follicular thyroid carcinoma | KRAS |
| 30% | Lung adenocarcinomas | One of the RAS |
| 30% | Myeloid leukaemia | One of the RAS |
| Many | Bladder cancers | HRAS |
| Many | Haematopoietic tumors | NRAS |
Sometimes, the Ras protein itself isn’t abnormal, but a protein in the downstream signalling cascade of Ras, like RAF or MAP kinase. Mutations in the RAF family is detected in 60% of all melanomas.
Non-receptor tyrosine kinases
Not all tyrosine kinases are part of receptors, we also have so-called nonreceptor tyrosine kinases (nRTKs). They have the same activity as their receptor counterparts (i.e. they phosphorylate tyrosine residues), but they’re cytosolic enzymes instead of intracellular components of a transmembrane receptor.
nRTKs are mainly involved in signal transduction in activated T and B cells. They’re involved in the signalling cascade of the T cell receptor, B cell receptor, IL-2 receptor and others. CD4 and CD8 also require nRTKs. It’s therefore not too hard to understand that nRTK mutations are most important in leukaemia.
Philadelphia chromosome
A gene called c-ABL codes for an nRTK in B-cells. In some unlucky individuals will a gene translocation of this gene occur, where it’s moved from chromosome 9 to chromosome 22, where it’s integrated into a gene called BCR (which is not the gene for the B cell receptor!). This t(9;22) change causes chromosome 22 to be defective and short, and contain a sort of “hybrid” BCR/ABL gene. This alteration is called Philadelphia chromosome, and is characteristic in chronic myelogenous leukaemia and acute lymphoblastic leukaemia.
The result of this translocation is that the new BCR/ABL gene will produce hybrid protein with an nRTK attached to it, which is always active and leads to unregulated cell division.
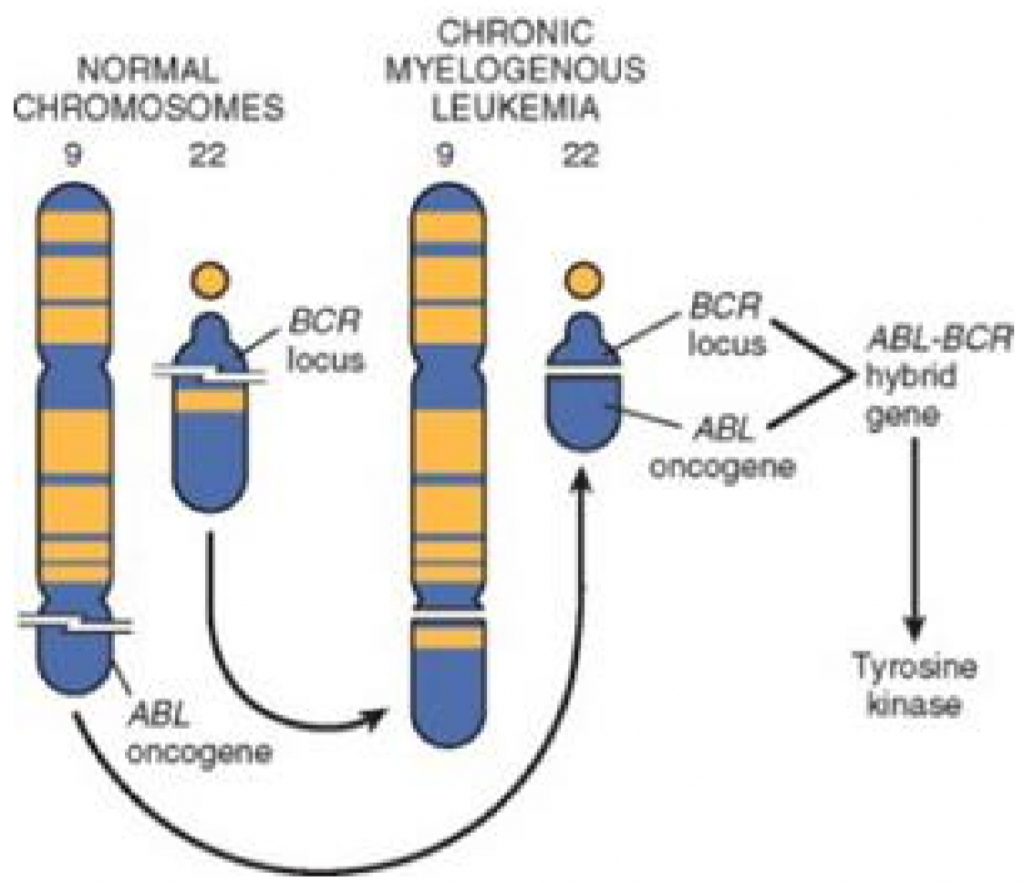
Philadelphia chromosome can be detected by karyotyping, FISH or PCR, and is a highly sensitive test for chronic myelogenous leukaemia.
Other important nRTKs
Two diseases, polycythaemia vera and primary myelofibrosis are caused by an activating mutation of an nRTK called JAK2, or Janus kinase 2.
HI! Under the Philadelphia chromosome you write B- cell receptor gene for BCR, its supposed to be Breakpoint Cluster Region:)
Glad i deg
Thank you so much! As soon as I saw the BCR abbreviation I just assumed it was the B-cell receptor. Thanks for clearing this up!
Glad i deg og!