Page created on March 21, 2019. Last updated on December 18, 2024 at 16:57
Introduction
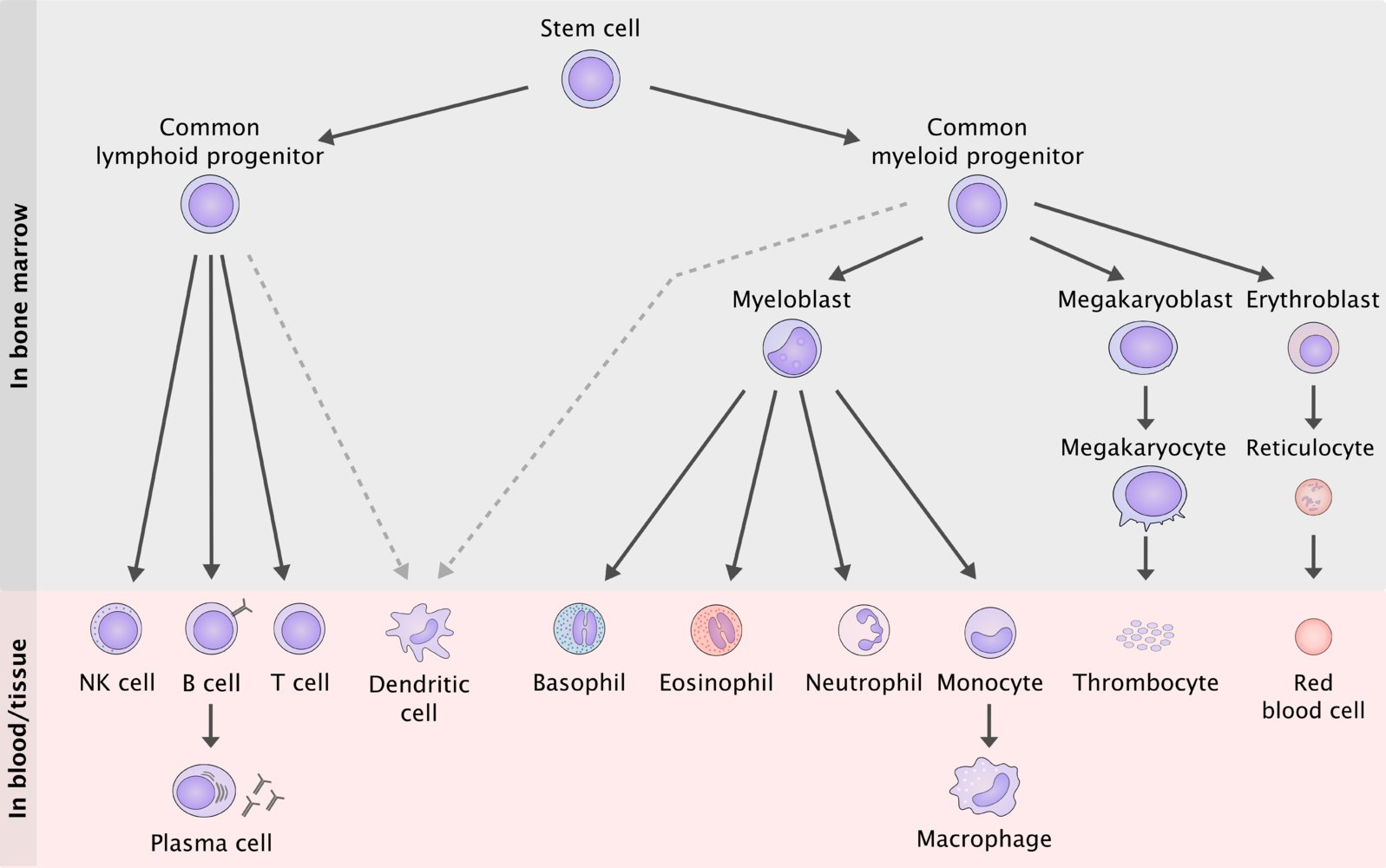 We now enter the world of myeloid neoplasms, which involves the myeloid cell lines on the right of the figure. In some myeloid neoplasms there are “blocks” or “defects” in the maturation of one or more cell lines. In other neoplasms myeloid progenitor cells have increased proliferation.
We now enter the world of myeloid neoplasms, which involves the myeloid cell lines on the right of the figure. In some myeloid neoplasms there are “blocks” or “defects” in the maturation of one or more cell lines. In other neoplasms myeloid progenitor cells have increased proliferation.
We divide the myeloid neoplasms into three subtypes, based on their inherent “defect”:
- Acute myeloid leukaemias – involves defect of cellular maturation and increased proliferation of precursors
- Myelodysplastic syndromes – involves defect of cellular maturation
- Myeloproliferative neoplasms – involves increased proliferation of precursors
Acute myeloid leukaemias
Acute myeloid leukaemias (AML) primarily affects older adults. It’s has multiple known risk factors:
- Ionizing radiation
- Chemotherapy
- Pesticides
- Genetic diseases
- Down syndrome
- Fanconi anaemia
AML involves a defect of the maturation of myeloid progenitors in the bone marrow and an increased proliferation of these precursors. The neoplastic cells are blocked at an early stage of myeloid cell development, so that they can’t differentiate into mature blood cells. These immature myeloid cells then accumulate in the bone marrow and experience increased proliferation. They replace the normal elements of the bone marrow and they frequently circulate in the peripheral blood, causing leukaemia.
These neoplastic cells may be:
- Myeloblasts
- Promyelocytes
- Monoblasts
- Promonocytes
- Megakaryoblasts
…and so on.
The definition of AML involves that more than 20% of the bone marrow must be comprised of these immature cells (“blasts”). The diagnosis is based on this and on the presence of characteristic genetic changes with the help of karyotyping and FISH.
Patients present with the symptoms of myelophthisis, i.e. pancytopaenia. Common symptoms involve weakness, fatigue, infections, pallor and bleeding.
The molecular pathogenesis of AML involves two different types of mutations. Type I mutations involve proteins in the signal transduction pathway (like RAS or JAK). Mutations of these proteins causes the cells to experience increased proliferation. Type II mutations involve proteins that are transcription factors (like RARA (retinoic acid receptor alpha)). These mutations cause the “block” in differentiation or causes the cells to differentiate abnormally. The development of AML is a multistep process that requires at least one type I mutations and one type II mutation.
A t(15;17) translocation, which fuses the proteins PML and RARA is present in only 10% of all cases of AML but is very important for the prognosis. This translocation breaks myeloid differentiation by reducing the normal function of retinoic acid receptors (RARA). The physiological level of vitamin A (retinoic acid) isn’t enough to cause these cells to differentiate when the retinoic acid receptors are defective.
Cases of AML with the t(15;17) translocation can be effectively treated with ATRA (all-trans retinoic acid) treatment. ATRA is a retinoic acid analogue that stimulates the defective retinoic acid receptors and therefore allows the tumor cells to differentiate properly.
Myelodysplastic syndromes
Myelodysplastic syndromes (MDS) are a group of haematological neoplasms that involves disordered or ineffective differentiation of the stem cells myeloid cell line. This leads to the appearance of dysplastic myeloid cell precursors, which partially or wholly replaces the bone marrow. There is no increased proliferation, just a defect in differentiation.
Blood and bone marrow cytology or histology shows signs of dysplasia. Dyserythropoiesis, dysgranulopoiesis and/or dysmegakaryopoiesis are common, leading to one or more cytopaenias. The bone marrow also shows a higher ratio of immature cells to mature cells.
MDS occurs in elderly with no apparent cause in 90% of cases. The remaining 10% of cases of MDS are secondary to chemotherapy, organic solvents or ionizing radiation.
The clinical picture is defined by the presence of one or more cytopaenias. Erythrocytopaenia is the most common cytopaenia, so symptoms of anaemia are most common.
MDS have a risk of progressing into acute myeloid leukaemia.