Table of Contents
Page created on March 30, 2019. Last updated on December 18, 2024 at 16:57
Late complications
We’ve seen the potential acute complications of DM, DKA and HHS. The late (long-term) complications of diabetes are:
- Macroangiopathy or macrovascular disease – disease of the larger blood vessels
- Caused by: Hypertension, atherosclerosis
- Coronary artery disease
- Cerebrovascular disease
- Peripheral artery disease
- Microangiopathy or microvascular disease – disease of the smaller blood vessels
- Diabetic retinopathy
- Diabetic nephropathy
- Diabetic neuropathy
- Diabetic cardiomyopathy
- Diabetic foot
- Increased tendency for infection
Let’s look at them in detail.
Hypertension
This is mostly characteristic for 2DM and occurs due to multiple mechanisms:
- Hyperinsulinism causes increased sodium reabsorption in the tubules
- Hyperglycaemia-induced hyperosmolarity causes more fluid to enter the intravascular space from other spaces
- Hyperinsulinism stimulates sympathetic activity and renin secretion
- Hyperinsulinism stimulates vascular smooth muscle cell proliferation
- Insulin resistance causes an increase in intracellular Ca2+ level in vascular smooth muscle cells, which increases their tone (source)
Atherosclerosis and macrovascular disease
The risk of atherosclerosis is increased in diabetes mellitus. It develops on the basis of these factors:
- Hyperlipidaemia
- Glycation of LDL
- Endothelial damage due to pseudohypoxia due to the polyol pathway
- Hypertension
- Chronic inflammation in obesity (mostly for 2DM)
The level of VLDL is high in DM as it’s synthesized in large amounts and its breakdown is inhibited (lipoprotein lipase is insulin-dependent). Oxidized and glycated LDL isn’t recognized by LDL receptors and will instead be taken up by scavenger cells, forming foam cells. VLDL is also phagocytosed by these cells.
The term macroangiopathy or macrovascular disease describes the condition where large vessels, like the coronaries, aorta, cerebral and peripheral arteries, are occluded by atherosclerotic plaques and/or thrombi. In almost all cases it’s caused by atherosclerosis. This can lead to:
- Coronary artery disease
- Angina
- AMI
- Cerebrovascular disease
- Stroke
- Peripheral artery disease – mostly affecting arteries of the leg
- Intermittent claudication – where walking causes pain
- Arterial insufficiency ulcers
- Gangrene
- Amputations
Unlike microvascular disease isn’t macrovascular disease strongly associated with chronic hyperglycaemia.
Microvascular disease
The small vessels of the retina, kidney and nerves are injured, causing diabetic retinopathy, diabetic nephropathy and diabetic neuropathy, respectively. The mechanism behind this involves pseudohypoxia (the polyol pathway), abnormalities of the PKC pathway, advanced glycation end-products (AGEs) and the hexosamine pathway. More details about this in the next topic.
The complications of microvascular disease typically occur 5 – 10 years after the onset of the disease. They occur due to chronic hyperglycaemia, meaning that they can be prevented or postponed by good glycaemic control.
Diabetic retinopathy
Diabetic retinopathy develops slowly and therefore occurs after around 15 years with the disease. It’s more common in those with 1DM than in 2DM. Diabetic retinopathy is the most common cause of adult blindness in developed countries.
Many factors contribute to diabetic nephropathy:
- Hypertension
- Destruction of pericytes
- Oxidative damage from reactive oxygen species
- Damage to the endothelium
It occurs in two stages:
- Simplex retinopathy / non-proliferative retinopathy
- Proliferative retinopathy
Simplex retinopathy / non-proliferative retinopathy: In this phase some people experience impaired vision and some people don’t, but changes are happening in the background which will later definitely impair vision. The alterations can be seen on the retina with an ophthalmoscope and include:
- Intraretinal microvascular abnormalities (IRMA) – remodelled arteries on the retina
- Microaneurysms
- Small haemorrhages
- Cotton-wool spots – fluffy spots of necrosis
- Macular oedema – which can cause visual loss
Proliferative retinopathy: This phase gets its name from how hypoxia of some parts of the retina induces proliferation of new capillaries. This neovascularization may cause bleeding that may cause the retina to detach, causing blindness. The bleedings themselves may also cause visual loss.
Cataract may also develop as the crystallin protein inside the lens is easily glycated.
Diabetic nephropathy
End-stage renal failure, caused by diabetic nephropathy, is a major cause of death in people with diabetes.
The pathomechanism involves a non-enzymatic glycation of the proteins of the glomerular basement membrane, which causes it to thicken and (paradoxically) have increased permeability. This causes hyperfiltration and increased filtration of proteins, both of which damage the glomeruli. Especially the mesangial cells respond to this by proliferating and producing more mesangial matrix, causing mesangial expansion. The mesangial matrix is replaced by fibrosis, causing glomerulosclerosis and formation of Kimmelstiel-Wilson nodules.
The timeline of diabetic nephropathy usually goes like this:
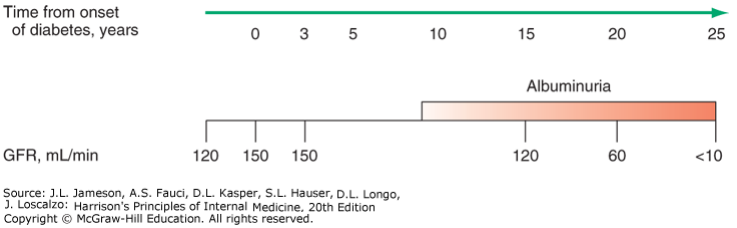
The colour intensity of the “albuminuria” bar shows how significant the albuminuria is.
The GFR actually increases (from ~120 to ~150 mL/min) after the onset of the disease. This hyperfiltration eventually damages the nephrons, causing chronic renal failure. The GFR eventually decreases, and the morphological changes allow proteins to be filtered as well. Very characteristic for diabetic nephropathy is that there is microalbuminuria (<300 mg/day) at first, which progresses into macroalbuminuria (>2 g/day).
Renoparenchymal hypertension also sets in as the renal parenchyme dies due to the chronic renal failure. In the end-stage of diabetic nephropathy there will be papillary necrosis.
Diabetic neuropathy
Diabetic neuropathy occurs in 50% of individuals with long-standing DM. Mostly autonomic and sensory nerves are damaged; motor functions are only partly affected. Common complications include distal symmetric polyneuropathy (affecting the distal parts of both feet) and autonomic neuropathy.
Distal symmetric polyneuropathy manifests as sensory loss, pain, numbness, tingling, burning, hyperesthesia or paraesthesia.
Autonomic neuropathy manifests as orthostatic hypotension, tendency for syncope, disorders of the GI motility, incontinence and anhidrosis. Disordered proprioception may cause ataxia.
These changes develop due to glycation and axon-degeneration of myelin proteins due to ischaemia and pseudohypoxia. Obstruction and other problems with capillaries that supply the nerves cause ischaemia. Ischaemia and pseudohypoxia decrease the activity of the Na+/K+ ATPase, which decreases the conduction velocity.
Deficiency of the pain sensation around the heart may contribute to the development of silent myocardial infarcts in diabetics.
Diabetic cardiomyopathy
Cardiomyopathy develops due to the increased atherosclerosis and coronary artery disease, hypertension, and potentially chronic lactic (?) acidosis.
Diabetic foot
DM is a leading cause of nontraumatic lower extremity amputation. Foot ulcers and infections are also major causes of morbidity in people with DM.
Diabetic foot develops on the basis of neuropathy, peripheral artery disease (atherosclerosis) and poor wound healing. Loss of sensation in the feet allows trauma to the food to go unnoticed and untreated. Ataxia may predispose to this trauma. Autonomic neuropathy causes anhidrosis, which promotes drying of the skin. Peripheral artery disease and poor wound healing (which is normal in DM) prevent small traumas from healing and instead allow them to enlarge and become infected. Deep, gangrenated ulcers are common.
These feet must be amputated to prevent sepsis.
Hei!
“microalbuminuria (2 g/day)”
These values kinda differs from patho 2 topic 21
The value should be < 300 mg/day. Fixed now.