Table of Contents
Page created on November 18, 2018. Last updated on December 18, 2024 at 16:56
Tuberculosis is a chronic granulomatous disease which usually involves the lungs, but may affect other organs as well, like the kidneys. The centre of the tubercular granulomas typically undergo caseous necrosis.
Epidemiology
Apparently, every third human is infected with tuberculosis. In 2016 there were 10,4 million new cases! And 1,5 million deaths due to the disease the same year. We in Europe are very lucky to live in an almost tuberculosis-free reality.
Also, tuberculosis is still a major cause of death worldwide. And it’s scary since the majority who have the disease have no symptoms of it.
Microbiology
Tuberculosis is caused by bacterium in the Mycobacterium Tuberculosis family. The mycobacteria are obligate aerobe bacteria, which means that they are dependent on oxygen to live. These bacteria are also capable of living both inside and outside cells and reproduce, therefore making them facultative intracellular pathogens. They proliferate slowly. Mycobacteriae are stained with Ziehl–Neelsen staining, also called acid-fast staining.
There are three different types:
1. M. Tuberculosis hominis
Most frequent type which is usually found in the lungs and cause an airborne infection.
2. M. Bovis
Is now very rare and is actually a pathogen of cows. This can cause oropharyngeal and intestinal tuberculosis if unpasteurized milk is drunk from a cow with tuberculosis.
3. M. Avium/Intracellulare
Doesn’t spread that easily since it almost only affects immunodeficient patients like patients with HIV.
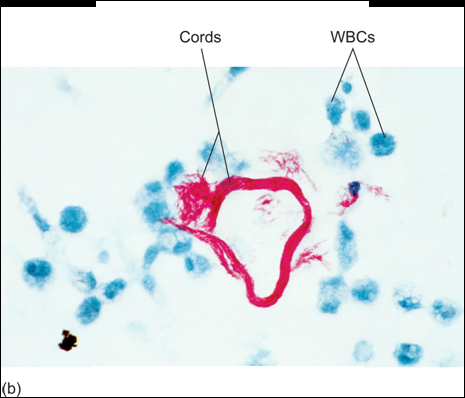
M. tuberculosis has two important compounds in its cell wall: cord factor and mycolic acid. Mycolic acid gives it defence against the complement system, free radicals and phagocytosis. Cord factor inhibits IFN-γ and activates TNF-α, which makes the inflammation worse. Cord factor also causes the bacteria to grow parallelly, which makes them look like “cords”. Only virulent (dangerous) strains of m. tuberculosis produce cord factor, so only virulent strains have the cord appearance.
These two molecules play an essential role in why the body can’t get rid of the bacterium easily.
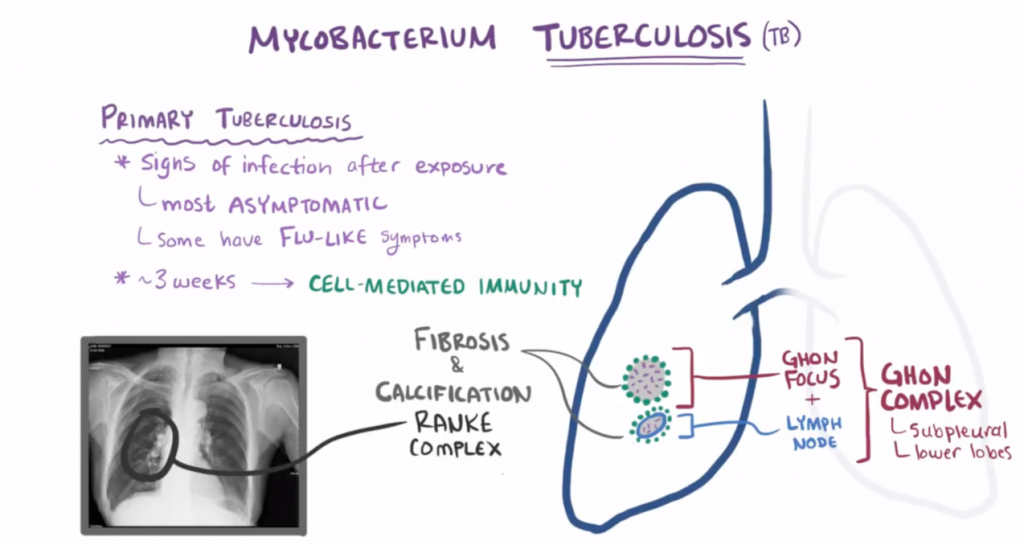
Primary tuberculosis
95 % of the cases are non-progressive primary tuberculosis. This affects persons who have been unexposed and unsensitized with a normal immune system.
Alveolar macrophages phagocytose the mycobacteria and try to digest them. However, the bacteria inhibit fusion of lysosomes with the phagocytic vacuole. This allows the mycobacterium to proliferate inside the macrophages. However, the macrophage will still present antigens of m. tuberculosis on its MHC II molecule. Despite the presence of bacteria in the body are the first 3 weeks usually asymptomatic or only present with flu-like symptoms.
Approximately 3 weeks after exposure, the alveolar macrophages with the antigen reach draining lymph nodes where CD4+ T-cells can be found. Macrophages then present the m. tuberculosis antigens to the CD4+ T-cells. This induces differentiation of TH cells to TH1 cells, which secrete IFN-γ. IFN-γ activates macrophages and recruitments more macrophages. Macrophages proliferate and become epithelioid cells, which is characteristic for the granulomatous response. These epithelioid cells fuse and make up giant cells, forming granulomas which traps the bacteria inside, preventing further spreading. The tissue in the core of the granulomas dies from caseous necrosis. In other words, the body sacrifices the parts of the lung to try to get rid of the bacteria.
This process is a form of type IV hypersensitivity.
Since the immune system traps the mycobacteria but can’t kill them will m. tuberculosis go into a latent state.
The area where the primary infection happened in the lungs is called the Ghon-focus. However, if tuberculosis spreads to the nearby lymph node and infects it too, will they together be called a Ghon-complex.
After healing, the fibrotic and calcified primary (Ghon-)focus together with the enlarged lymph node is called the Ranke-complex. Ranke-complex is the “ending” of primary non-progressive tuberculosis. However, mycobacterium tuberculosis can survive inside the Ranke-complex for many years. The patient is now sensitized.
5 % of the cases of primary tuberculosis will develop into the primary progressive tuberculosis. This occurs in immunodeficient persons. The immune system in these cases cannot create granulomas and trap the bacteria. The bacteria can therefore do the following:
- Spread with the blood (haematogenous dissemination) and cause:
- Miliary tuberculosis
- Basilar meningitis, an infection on the base of the brain
- Landouzy sepsis if the patient already has AIDS. The process is fulminant (quick) and causes death.
- Spread through the bronchi (bronchogenous dissemination) and cause:
- Caseous pneumonia and pleuritis
- Primary cavity formation (Pthisis cavernosa/pulmonis)
- Pleural effusion
- Epituberculosis
- Spread through the lymphatic system (lymphogenous dissemination)
- Scrophulosis (mycobacterial cervical lymphadenitis)
Post-primary tuberculosis
Post-primary tuberculosis occurs when a previously sensitized host is re-infected or reactivated due to immune deficiencies or old age.
Endogenous reactivation occurs when the m. tuberculosis bacteria that are latent in the Ranke-complex are reactivated. This happens if a patient with Ranke-complex develops an immune system deficiency, or experiences trauma or surgery.
After becoming reactivated can the m. tuberculosis bacteria spread with the blood and infect any organ in the body, but especially the genitals, kidneys (phthisis renalis), vertebrae (spondylitis) and serous membranes.
Exogenous re-infection occurs when a patient with Ranke-complex again becomes infected from the outside (not by the bacteria inside the Ranke-complex). The morphology in exogenous re-infection is different from endogenous reactivation. Here will the lungs mainly be affected. Re-infection usually begins in the apex of the lung and, as it progresses, will the infection destroy the lung parenchyme. This descending infection is called Assman infiltrate. The destroyed parenchyme leaves behind a cavity. If the cavity is just in the apex do we call it a secondary cavity. If the cavity is anywhere else in the lung do we call it phthisis pulmonus.
Extrapulmonary tuberculosis
With hematogenous spread, the tuberculosis can end up in other organs than the lungs. This occurs in both primary progressive TB and post-primary endogenous reactivation. Let’s take a look at the different organs and the consequences for them.
- Central nervous system
- Basilar meningitis. Affects especially children.
- CNS tuberculoma.
- Bone tuberculosis
- Pott’s disease which leads to compression of vertebrae.
- Kidney
- Miliary kidney tuberculosis in the cortex of kidneys
- Phthisis renalis where it forms cavities in the kidney.
- GI-tract
- Often from M. bovis infection.
- Gives ulcers, bleedings, malabsorption etc.
- Skin
- Other organs
- Adrenal glands
- Epididymis
- Testis
- Uterus
- Ovaries
- Fallopian tubes
Screening and vaccination
You are probably familiar with the BCG vaccine used against tuberculosis. However, its effect is discussable. Its protection against miliary tuberculosis and meningitis tuberculosa is good, but it doesn’t protect against post-primary tuberculosis.
The Mantoux test is a test containing tuberculin. It’s injected to the subcutis, and 48 hours later, an induration can be measured if the test is positive. However, this will be false positive with the BCG vaccine.
Lung screening is a safe way of preventing spread of tuberculosis.
For this topic maybe it’s also nice to mention the Ziehl– Neelsen Staining ,my seminar teacher specified it. But I guess it’s not a big deal if you forget ! 🙂
Good idea, thank you!