Table of Contents
Page created on November 18, 2018. Last updated on November 22, 2019 at 20:13
A chronic inflammation is prolonged, and lasts for weeks, months or even years! Here, inflammation, tissue injury and healing proceed simultaneously.
It differs from acute inflammation because there are no vascular changes, oedema and neutrophilic infiltrate. The characteristics of chronic inflammation are:
- Infiltration with mononuclear cells like macrophages, lymphocytes and plasma cells.
- Tissue destruction
- Repair involving angiogenesis and fibrosis.
Causes
A prolonged acute inflammation that cannot be resolved develops into a chronic inflammation. Other causes include:
- Inflammatory bowel disease
- Viral infections
- Foreign materials or pathogens that are difficult to get rid of
- Silica crystals (silicosis)
- Tuberculosis
- Hypersensitivity reactions
- Tuberculosis
- Rheumatoid arthritis
- Allergies
A loss of balance between pro- and anti-inflammation is always present in chronic inflammation.
Cells in chronic inflammation
As mentioned earlier, the cells dominating in a chronic inflammation are monocytes/macrophages and lymphocytes. Fibroblasts are also involved in the repair prosses. Let’s take a closer look at each of them.
- Macrophages
Macrophages are the dominant cell type in chronic inflammation. When monocytes arrive to a site of inflammation, they differentiate into macrophages. Macrophages are scattered all over the body and have different names that tell us where they are found.
- Histiocytes are found in the spleen and lymph nodes
- Kupffer-cells belongs to the liver
- Microglial cells are found in the CNS
- Alveolar macrophages are found in the alveoli
They are all major cells when it comes to reparation and chronic inflammation as they secrete cytokines and phagocytose materials. They are all part of the mononuclear phagocyte system. Recall that macrophages are antigen-presenting cells and have MHC II and are therefore able to present antigens to CD4+ T-cells.
Macrophages can become activated in two ways. If a macrophage is activated by microbes and IFN-γ will it be activated by the classical pathway and become an M1 macrophage. M1 macrophages are pro-inflammatory, they phagocytose and secrete lysosomal enzymes, reactive oxygen species and NO, and pro-inflammatory cytokines.
If a macrophage is activated by IL-13 and IL-4 (anti-inflammatory cytokines) will it be activated by the alternative pathway and become an M2 macrophage. M2 macrophages are involved in tissue repair and anti-inflammation. M2 macrophages don’t phagocytose but produce growth factors and anti-inflammatory cytokines like TGF-β and IL-10.
In chronic inflammation are both M1 and M2 macrophages present, as they both fight to inflame and repair the same tissue.
- Lymphocytes
They are the major cells of the adaptive immunity and play also an important role in chronic inflammations. Both T and B cells migrate to the site of inflammation.
The T-cells gets activated by cytokines produced mainly by macrophages. Remember that macrophages display antigens on its MHC II molecule. By this and secretion of IL-12, the T-cells begin to secrete their own cytokines like IFN-γ, which activates more M1 macrophages.
B-cells may differentiate into plasma cells to produce antibodies. Of the T-cells are the CD4+ T-cells most important. TH1 cells and TH17 cells secrete pro-inflammatory cytokines, which recruit and activate macrophages, while TH2 cells secrete anti-inflammatory cytokines which activate M2 macrophages.
- Fibroblasts
Fibroblasts are responsible for production and remodelling of collagen and extracellular matrix. They repair by activating angiogenesis, restoring the tissue damage done by the inflammation.
- Eosinophils
Recall from immunology and physiology that eosinophils are present if there is a parasitic infection or an IgE mediated immune response, like in hypersensitivity type I.
In chronic inflammation will M1 macrophages and inflammatory TH cells activate each other continuously to propagate the chronic inflammation. Macrophages present antigens to and stimulate TH-cells, which stimulates the TH-cells to produce inflammatory cytokines that recruit and activate more M1 macrophages, and so on. The continuous cycle fuels and sustains the chronic inflammation.
Mediators of chronic inflammation
Fibrosis and healing-mediating mediators
- Transforming growth factor, TGF-β
This one is produced by many cells but is found in its latent form until it gets activated by either plasmin or activated macrophages.
TGF-β has an anti-inflammatory and fibrosis-enhancing effect.
- Platelet derived growth factor, PDGF
Stimulates fibroblasts and smooth muscle cells.
- Fibroblast growth factor
Produced mostly by the fibroblasts. It stimulates endothelial cell proliferation, macrophage and fibroblast activation.
- Vascular endothelial growth factor, VEGF
Many cells produce this in response to hypoxia or the presence of PDGF and TGF- β. It is the most important factor when it comes angiogenesis.
- Pro-inflammatory mediators
- TNF-α
- IFN-γ
- IL-1
- IL-12
Examples of chronic inflammation
Chronic gastritis is the inflammation of the mucosa in the stomach, and can be caused by:
- Heliobacteria pylori
- Autoimmune gastritis
- Bile reflux
- Caffeine
- Alcohol
- Smoking
Helicobacter pylori is a gram-negative bacterium that lives in the antrum of the stomach and causes hyperacidity. More than 50 % of the global population have it, but not everyone has symptoms. It is also known to increase the risk of peptic ulcers, gastric lymphoma and gastric adenocarcinoma.
Rheumatoid arthritis is a chronic inflammation that is most frequent in women between 20 and 40 years. It is an autoimmune disease that affects joints and is caused by the type IV hypersensitivity reaction.
TH1 and TH17 cells are important. Several auto-antibodies can be found in the serum of patients with RA, like anti-CCP antibodies or Rheumatoid factor, an anti-IgG antibody. However, not all patients with RA have antibodies in their sera at all, meaning that both cellular and humoral immune response are important in the disease.
Common symptoms are:
- Symmetrical polyarthritis (swelling of joints)
- Vasculitis
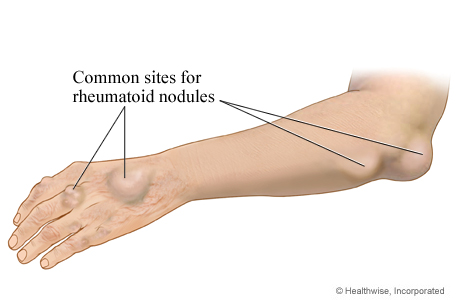
- Rheumatoid nodules
- Hard nodules under the skin
- Amyloidosis and lung fibrosis (rare)
The treatment for RA isn’t curative but instead tries to slow the progression of the disease. NSAIDs and/or steroids are used the whole life.
Hello,
I have one question. In the part of the lymphocytes in cell chronic inflammation it says that the T cells secret tnf gamma. The question is the following :does that exits?or its inf gamma or tnf alpha?
Best regards
Hey! No, it doesn’t exist. It should be IFN gamma. Corrected now.
The next time I read a blog, I hope that it doesnt disappoint me as much as this one. I mean, I know it was my choice to read, but I actually thought youd have something interesting to say. All I hear is a bunch of whining about something that you could fix if you werent too busy looking for attention.
I laughed so hard at this
Miss Luetta Perney? You good?
How is TNF-alpha a pro-inflammatory mediator?
I’m not sure how to answer the question. Do you imply that it is actually an anti-inflammatory cytokine?
TNF-alpha is a pro-inflammatory cytokine that is activated by the transcription factor NF-kB: https://greek.doctor/medical-biochemistry/lectures/22-oxidative-stress-induced-signaling-pathway/ . It is one of the three cytokines that activates the acute phase reaction: https://greek.doctor/immunology/lectures/6-inflammatory-reaction/
no no, I didn’t mean to imply anything! But I understand now after looking at the links you posted! thanks
I’m happy to help!