Table of Contents
Page created on October 18, 2018. Last updated on December 18, 2024 at 16:57
| Drugs influencing catecholamine biosynthesis | Drugs influencing noradrenaline storage | Indirectly acting adrenergic drugs | Noradrenergic neuron blocking agents |
| Benserazide | Reserpine | Cocaine | Bretylium |
| Carbidopa | Tetrabenazine | Ephedrine | Guanadrel |
| Levodopa | Amphetamine | Guanethidine | |
| Methyldopa | Tyramine | ||
| Methyltyrosine |
Noradrenaline
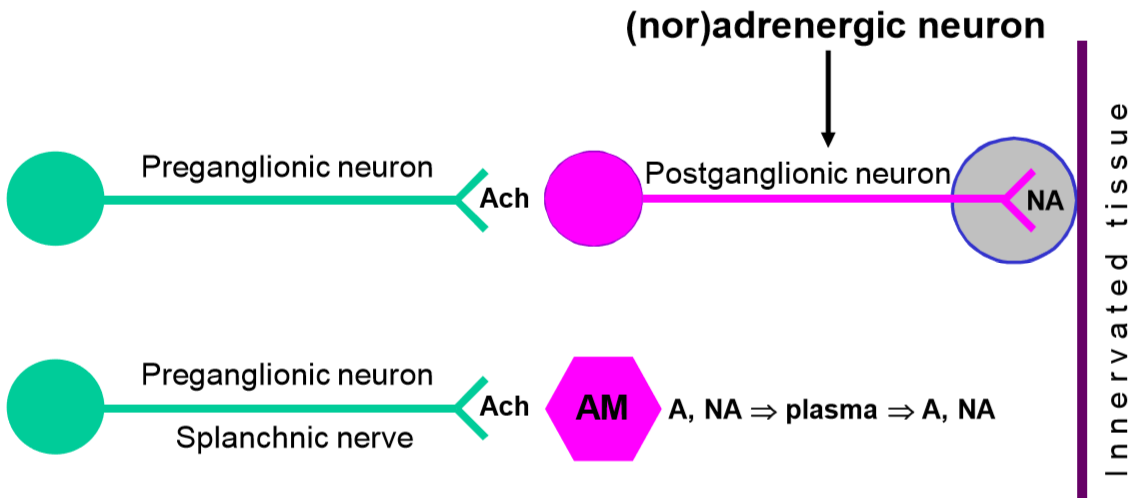
 In the sympathetic nervous system, preganglionic neurons activate postganglionic neurons with acetylcholine, and postganglionic neurons activate the innervated tissues with noradrenaline. In other words, noradrenaline is the postganglionic neurotransmitter in the sympathetic nervous system. By controlling the release and function of noradrenaline, we can block or enhance certain functions of the sympathetic nervous system.
In the sympathetic nervous system, preganglionic neurons activate postganglionic neurons with acetylcholine, and postganglionic neurons activate the innervated tissues with noradrenaline. In other words, noradrenaline is the postganglionic neurotransmitter in the sympathetic nervous system. By controlling the release and function of noradrenaline, we can block or enhance certain functions of the sympathetic nervous system.
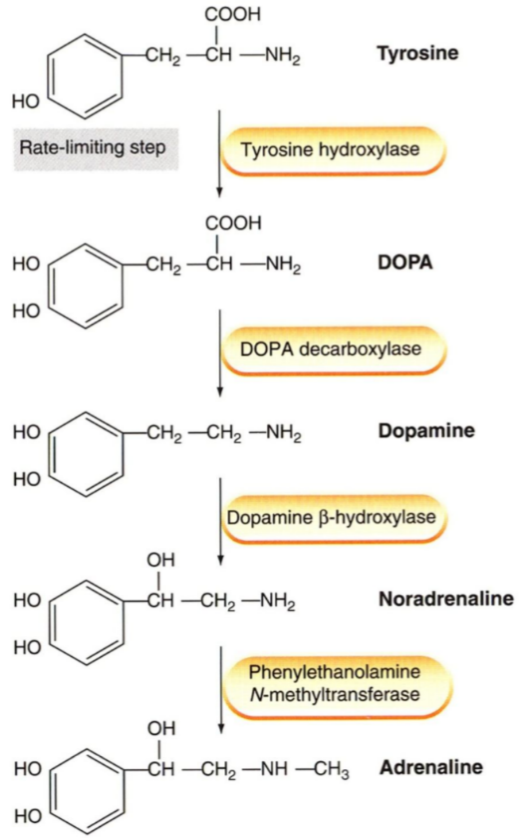
As learned in biochemistry, the catecholamines (dopamine, noradrenaline and adrenaline) are synthesized from tyrosine.
Mechanism of release:
The presynaptic neuron takes up tyrosine through a transporter, the tyrosine is hydroxylated to DOPA, which is decarboxylated into dopamine. Dopamine is then transported into a vesicle by an important transporter called VMAT. Inside the vesicle dopamine is hydroxylated into noradrenaline. The vesicles now contain noradrenaline and dopamine β-hydroxylase. The vesicle waits at the nerve ending for an action potential to arrive.
When an action potential arrives it will cause voltage-gated Ca2+ channels to open, causing an influx of Ca2+ into the cell. This triggers exocytosis of the vesicle, causing it to release its content into the synaptic cleft. In the synaptic cleft the released noradrenaline can have three different fates:
- It can diffuse over the synaptic cleft to bind to postsynaptic noradrenaline receptors, which carry the signal to the innervated tissue.
- It can be recycled into the presynaptic nerve ending by norepinephrine transporter (NET). NET is also called uptake-1.
- Lastly, it can bind to special inhibitory α2-adrenergic receptors on the presynaptic nerve ending.
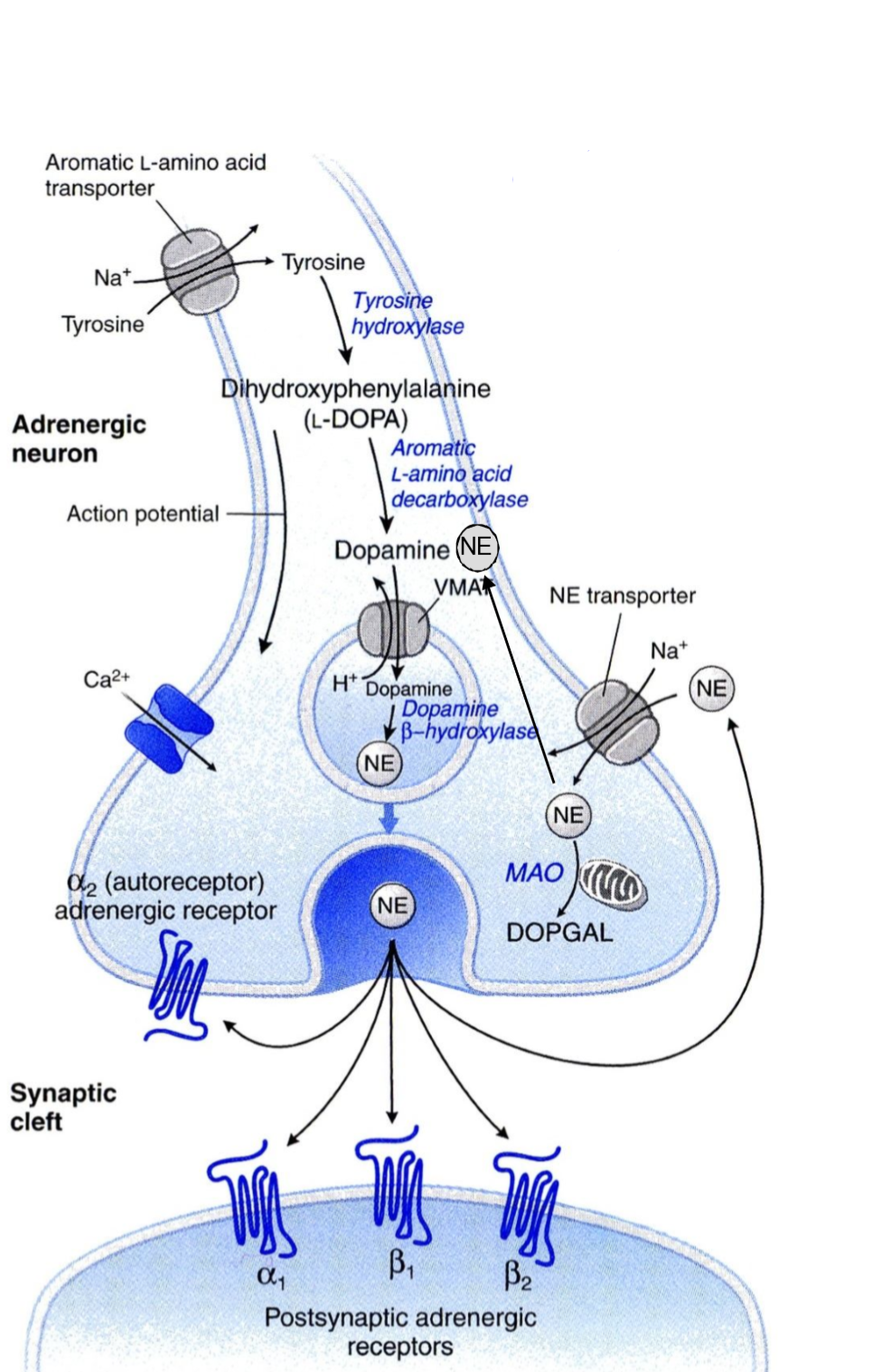
The α2-adrenergic receptors work as a negative feedback mechanism. When noradrenaline released from the nerve ending binds to the α2-receptors (on the same nerve ending) adenylyl cyclase inside the axon will be inhibited, which inhibits further NA release.
Elimination:
Monoamine oxidase (MAO) will degrade catecholamines into a compound called DOPGAL.
Aldehyde reductase and COMT are involved in the breakdown of DOPGAL into the final end-products to be excreted in urine.
Drugs influencing catecholamine synthesis
- Methyltyrosine
- Levodopa (L-DOPA)
- Carbidopa
- Benserazide
- Methyldopa
Indications:
Methyltyrosine is used in the therapy of phaeochromocytoma.
Levodopa, carbidopa and benserazide are used in the therapy of Parkinson disease.
Methyldopa is used to treat hypertension in pregnancy.
Mechanism of action:
Methyltyrosine is a false substrate for the tyrosine hydroxylase enzyme, that usually uses normal tyrosine. Methyltyrosine will inhibit the enzyme, which inhibits the noradrenaline and adrenaline synthesis.
Dopamine is deficient in Parkinson’s, so increasing dopamine in the CNS is essential. Dopamine doesn’t cross the blood-brain barrier, however L-DOPA, dopamine’s precursor does, so we give that instead. It often given with carbidopa or benserazide.
Carbidopa and benserazide are DOPA decarboxylase inhibitors. They don’t cross the blood-brain barrier either, so when we give either of them together with levodopa we can ensure that the levodopa isn’t converted into dopamine before it reaches the CNS.
Methyldopa is a false substrate for DOPA decarboxylase. The enzyme converts it to methyldopamine, which is converted by dopamine β-hydroxylase into methylnoradrenaline. Methyl-NA has a high effect on α2-receptors and little effect on α1-receptors, meaning that it increases the negative feedback. Also, as a false substrate methyldopa will reduce the capacity DOPA carboxylase has to convert normal DOPA into dopamine, which reduces the amount of noradrenaline formed.
Drugs influencing noradrenaline storage
Reserpine is an irreversible inhibitor of VMAT. This causes dopamine and noradrenaline to remain in the cytosol instead of entering the vesicle. They will therefore be degraded by MAO. The end effect is that reserpine depletes NA and dopamine in the nerve ending. It’s not used clinically anymore.
Tetrabenazine is a selective VMAT2 inhibitor used to treat Huntington’s chorea. VMAT2 is a type of VMAT only found in the CNS.
Indirectly acting sympathomimetic agents
- Ephedrine
- Non-drugs
- Cocaine
- Amphetamine
- Tyramine
Indications:
Ephedrine is used to treat nasal congestion, haemorrhoids, hypotension, nocturnal enuresis and to produce mydriasis.
Amphetamine and cocaine are abused for their strong psychomotor effects and appetite-reducing effects.
Mechanism of action:
These drugs enter the adrenergic nerve ending by acting as false substrates for NET. They are also taken up into the vesicles by VMAT. This causes noradrenaline to be leave the cytosol into the synaptic cleft through NET.
Cocaine doesn’t work the same way. It instead inhibits the NET altogether, so that noradrenaline cannot be removed from the synaptic cleft. This causes the effect of noradrenaline to last for much longer.
Ephedrine is also a direct β receptor agonist.
Pharmacokinetics:
Tachyphylaxis often develops, as the pool of noradrenaline inside the cytosol of the presynaptic neuron quickly empties.
Cheese reaction:
The cheese reaction is a reaction which occurs when a person who takes MAO-A inhibitor drugs (antidepressants) consumes high amounts of a compounds called tyramine. Tyramine is an indirect sympathomimetic which is found in high amounts in red wine and certain types of cheese.
MAO-A normally breaks down ingested tyramine in the GI tract and liver. However, when this enzyme is inhibited tyramine will not be broken down, so it can cause severe hypertension.
Noradrenergic blocking agents
- Bretylium
- Guanethidine
- Guanadrel
Indications:
These drugs are no longer used clinically.
Mechanism of action:
These drugs enter the presynaptic noradrenergic neurons by serving as alternative substrates for NET. Inside the cytoplasm will they block the voltage-gated Na+ channels, thereby preventing the propagation of a nerve signal along the neuron and therefore inhibiting the exocytosis of the vesicles containing noradrenaline.
The overall effect is the inhibition of physiological, exocytotic noradrenaline release. These drugs are therefore sympatholytic in action.
Adverse effects:
While extremely effective in lowering standing blood pressure, these drugs had several significant drawbacks. They did not lower BP in lying position, and they caused severe postural hypotension, diarrhoea, nasal congestion and failure of ejaculation.
Hey!
Isn’t methyltyrosine inhibitory drug?
How can it increase synthesis of NA?
Does it say anywhere on the page that methyltyrosine increases synthesis of NA? I can’t find it.
Bensarazide under DRUGS INFLUENCING CATECHOLAMINE SYNTHESIS and Banserazide in the introduction, are they misspelling of benserazide?
You’re quite sharp with grammar. I’ve corrected the misspellings.
Hey Nik, shouldnt the postganglionic neuro take up the tyrosin as biosynthesis of noradrenaline happens in the postganglionic neuron ?
Nice catch! You’re right, it’s the postganglionic neuron which takes up tyrosine. However, the word I meant to write there was presynaptic neuron. Corrected now.
Thanks ! Keep up the good work 🙂
Hi!
Mechanism of release:
The PREgaglionic neuron that takes up the the tyrosin
Yes