Table of Contents
Page created on March 14, 2018. Last updated on December 18, 2024 at 16:55
Learning objectives
- What are the four levels of protein structure?
- Why is protein folding a spontaneous process?
- What are chaperones?
- What are heat shock proteins?
- What is the GroEL/GroES complex?
- What are nucleoplasmins?
- Which enzymes are involved in protein folding?
- How is cystic fibrosis related to protein folding?
Protein structure
Proteins are synthesized as one-dimensional polypeptide chains, but end up as three-dimensional. This process is called protein folding. Correct folding, that is, correct manipulation of the polypeptide chain so it created the correct three-dimensional protein, is essential for its function.
Proteins have four “levels” of structure. The primary structure of the protein refers to the sequence of amino acids in the polypeptide chain. The secondary structure refers to the α-helixes and β-sheets the polypeptide chain forms. The tertiary structure refers to the three-dimensional from the protein takes when the α-helixes and β-sheets form a globular structure. The quaternary structure refers to the three-dimensional structure which consists of multiple polypeptide chains, known as subunits. These subunits are products of different genes but act as a single functional unit.
The structure of the protein is largerly dependent on the amino acid sequence, but it is also influenced by its environment.
Protein folding
To understand protein folding, we need to remember thermodynamics. Molecules always want to have as little free energy as possible. The less free energy (entropy) a molecule has, the more stable it is. Proteins are correctly folded when they are in a conformation that contains as little free energy as possible. Because a folded protein contains less free energy than an unfolded protein, protein folding is a spontaneous process. However, the proteins might still need help sometimes.
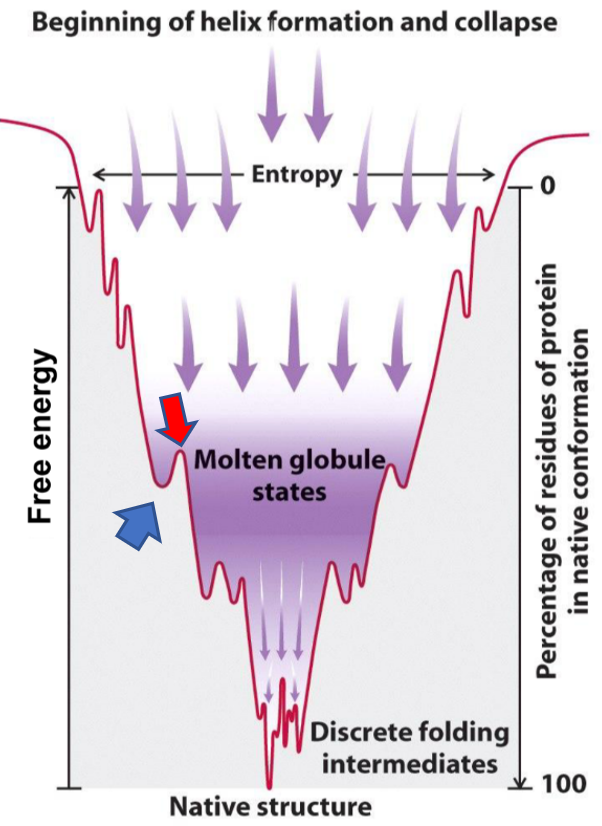
- The thermodynamics of protein folding. Note that when the protein is in the lowest point of the canyon is when it is in its native, active form.
Consider the picture above. The protein starts at the top of the canyon, when it is an unfolded polypeptide chain and contains a lot of free energy. When it begins to fold by itself, it will lose some of this free energy, become more stable, and fall down the canyon. However, the protein might get stuck at some places. Let’s say the protein starts to fold, but gets “stuck” where the blue arrow points. The protein still contains a lot of free energy (it’s only halfway to the bottom!), but it cannot continue to fold, because of the little “energy peak” that the red arrow points to. The protein needs a little energy “push” to get over this peak, to continue folding correctly to finally fold into its native, active structure. This “push” can be provided by chaperones.
Another example you can use to imagine why proteins fold, is to look at your hand. To stretch it open to a flat hand, you need to use some energy. If you let go, the hand will spontaneously “fold” because this “folded” state is the one that requires the least energy.
Sloth fun fact: the human hand is relaxed in an unfolded state, but the sloth hand is relaxed in a folded state. That means that sloths must use energy to open their hands. This explains how they can keep holding the tree while sleeping.
Chaperones
Molecular chaperones are molecules that catalyse the correct folding of proteins. Some chaperones are always present in the cell, while some are expressed only in response to cellular stress.
During cellular stress, like heat, proteins have a higher tendency to fold incorrectly. In these cases, chaperones are even more needed than usual. That’s why cellular stress increases expression of chaperones are called heat shock proteins (hsp).
Chaperonins are a class of chaperones. The best-known chaperonin is the GroEL/GroES complex, which is found in bacteria. The eukaryotic heat shock proteins Hsp60 and Hsp10 are structurally and functionally nearly identical to GroEL and GroES, respectively. The GroEL/GroES complex is a tube-formed structre which unfolded proteins will enter and fold inside.
Nucleoplasmins are chaperons which assist in the assembly of chromatin.
Some enzymes are also required for correct protein folding. These enzymes modify the primary chemical bonds in proteins, creating more favourable thermodynamics for correct folding. These enzymes include protein disulfide isomerase and peptide prolyl cis-trans isomerase.
Protein misfolding
Misfolded proteins are involved in the pathogenesis of many diseases, including cystic fibrosis, Parkinson disease, Alzheimer disease, and Huntington disease.
Cystic fibrosis is a disease characterised by a defective chloride ion channel called CFTR. People with this disease have a mutation in the CFTR gene, which causes the protein to misfold and be degraded instead of deployed to the cell membrane, or a misfolded and therefore dysfunctional CFTR protein is deployed to the cell membrane.
Prions
Prions are misfolded proteins which cause other proteins to misfold upon contact. Diseases where prion disease are involved, like mad cow disease and Creutzfeld-Jakob disease, are fatal in almost 100% of cases. Misfolded proteins accumulate and create fibrillar protein aggregates called amyloids, which damage the brain.
Summary
- What are the four levels of protein structure?
- The primary structure of the protein refers to the sequence of amino acids in the polypeptide chain.
- The secondary structure refers to the α-helixes and β-sheets the polypeptide chain forms.
- The tertiary structure refers to the three-dimensional from the protein takes when the α-helixes and β-sheets form a globular structure.
- The quaternary structure refers to the three-dimensional structure which consists of multiple polypeptide chains, known as subunits. These subunits are products of different genes but act as a single functional unit.
- Why is protein folding a spontaneous process?
- The protein has the least internal entropy when in its correct folded state
- What are chaperones?
- Chaperones are molecules which help proteins fold correctly
- What are heat shock proteins?
- Heat shock proteins are chaperones which are activated in cases of cellular stress, like heat
- What is the GroEL/GroES complex?
- GroEL/GroES complex is a chaperonin with a tube-formed structre which unfolded proteins will enter and fold inside
- What are nucleoplasmins?
- They are chaperons which assist in the assembly of chromatin
- Which enzymes are involved in protein folding?
- Protein disulfide isomerase and peptide prolyl cis-trans isomerase
- How is cystic fibrosis related to protein folding?
- In cystic fibrosis a chloride ion channel called CFTR is misfolded, causing it to be absent or dysfunctional