Table of Contents
Page created on February 9, 2019. Last updated on January 7, 2022 at 22:04
Growth factors
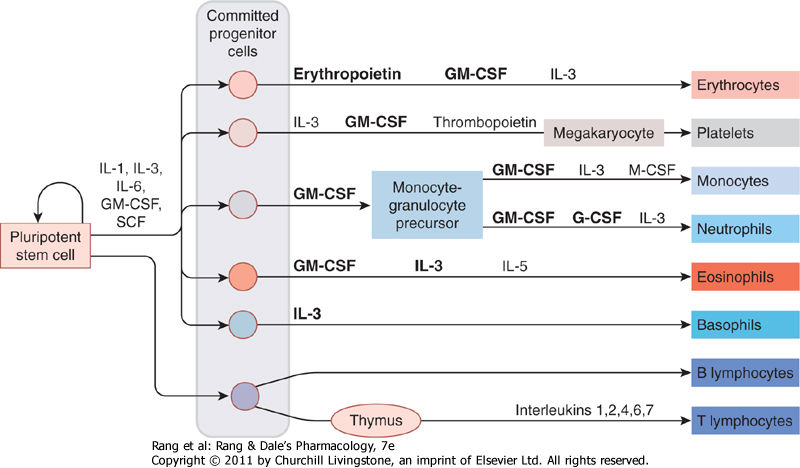
The normal haematopoiesis depends on the presence of certain growth factors. Increased level of these growth factors stimulates certain cell-lines, as seen in the figure above. By administering these growth factors can we increase the levels of certain cells if their levels are lacking. The growth factors that are bolded are the ones we give clinically.
The most important ones are:
- GM-CSF (granulocyte-macrophage colony stimulating factor) – stimulates erythrocyte, platelet, monocyte, neutrophil and eosinophil production
- G-CSF (granulocyte colony-stimulating factor) – stimulates only neutrophil production
- EPO (erythropoietin) – stimulates only erythrocyte production
- TPO (thrombopoietin) – stimulates only platelet production
We can understand that we can treat low levels of different blood cells by administering different growth factors. The growth factors are small glycoproteins so that can be artificially synthesized without too much problem.
Growth factors clinically
Recombinant EPO: Epoetin is the drug form of erythropoietin. Don’t be misled – they’re the exact same molecule, however we use the term EPO when it is endogenously produced and epoetin when it’s exogenously produced in cell cultures. The half-life of epoetin is short, so an alternative drug also exists which has longer half-life. Darbepoetin is the same molecule as EPO except it’s very glycosylated. This gives it longer half-life without impairing its function.
Recombinant EPO are mostly given intravenously or subcutaneously and can’t be given orally. This applies to the other growth factor analogues as well. The clinical indications for EPO analogues are:
- anaemia associated with chronic renal failure (where EPO production is impaired)
- anaemia that occurs due to chemotherapy
- to prevent anaemia that occurs in premature infants
Recombinant EPO may have the following side effects:
- Flu-like symptoms – due to the immune reaction against the drug
- Iron deficiency – as the increased RBC production depletes the iron storage
- Increased blood viscosity -> hypertension, headache
Recombinant G-CSF: Filgrastim is the drug form of G-CSF. Lenograstim is the glycosylated and therefore longer-lasting form, while pegfilgrastim is “pegylated” and has the longest duration of action of the three. These drugs stimulate the production of neutrophils.
These drugs are indicated in:
- neutropoenia due to cytotoxic drugs
- to harvest progenitor cells
- in aplastic anaemia
- in persistent neutropoenia in HIV infection
Recombinant GM-CSF: Molgramostim and the glycosylated form sagramostim stimulate the production of all granulocytes, not only neutrophils.
They’re indicated in severe granulocytopaenias.
Potential side effects of G-CSF and GM-CSF analogues are:
- Fever
- Rash
- GI disorders
- Bone pain
Recombinant TPO: Eltrombopag is not the same molecule as TPO; instead is it a TPO receptor agonist, so it elicits the same effect as TPO. Romiplostim isn’t the exact same as TPO either, it’s a fusion protein of TPO and another protein. Oprelvekin is the same as IL-11, which also stimulates platelet production.
They’re indicated in severe thrombocytopaenia.
Iron
You’ve been told this many times now: Iron is important for many enzymes in our body, as well as haemoglobin and myoglobin.
Iron is consumed in foods like meat and spinach. The daily requirement is 5mg for men, 15mg for children and menstruating women and 30 – 150mg for pregnant women. Iron is consumed in the oxidized Fe3+ form, called ferric iron. However, iron can only be absorbed in the ferrous Fe2+ form.
Iron circulation: Iron is absorbed mostly in the duodenum and jejunum. The absorption from the intestinal lumen into the intestinal epithelial cells is rapid, unregulated and occurs by diffusion. The transport from the intestinal epithelium into the circulation is tightly regulated to prevent overabsorption of iron. If the body doesn’t need more iron will the iron remain in the intestinal epithelium. This is called the epithelial barrier.
Ferric iron (Fe3+) travels with the food until it meets the brush border, where it is reduced to ferrous iron (Fe2+), which then enters the epithelial cell. Inside the cell is ferrous iron oxidized back to ferric iron and bound to apoferritin to form ferritin and is stored in the ferritin form. Iron will not be released from ferritin unless the body needs iron.
If the iron stores in the liver are full will the liver produce hepcidin, which travels to the intestinal mucosa and degrades ferroportin, the transporter that would allow iron to be transported from the intestinal epithelium to the circulation. When the iron stores are not full will the liver produce less hepcidin which results in ferroportin not being degraded and allowing iron to be transported to the circulation.
Inside the circulation is ferric iron (Fe3+) bound to transferrin. In normal situations is only 30% of the transferrin in the blood saturated with iron, so the transferrin saturation is normally 30%.
Iron is stored in our body, mostly in the liver, in the form of ferritin inside cells. A considerable amount is also stored in the intestinal mucosa. Ferritin is also present in the plasma. The plasma level of ferritin is proportional to the total level of ferritin in our body – the plasma ferritin level can therefore indicate whether the iron stores are low or high.
Iron is lost from our body in very small amount – only about 1 mg per day. This loss is mostly due to intestinal mucosal cells dying and due to desquamation.
Iron – clinically
Iron administration: Iron deficiency causes anaemia as there isn’t enough iron to produce haemoglobin for the RBCs. Iron deficiency anaemia is microcytic and hypochromic due to the lack of haemoglobin. In these cases we should administer iron to the patient.
The causes for iron deficiency include:
- Insufficient intake
- Chronic blood loss – cancer, menorrhagia
- Increased requirement – pregnancy, infancy
- Inadequate absorption
Iron tablets can be administered orally. These tablets contain iron in the more absorbable ferrous (Fe2+) form, often as a salt with sulphate, succinate or others. It can also be administered parenterally by intramuscular or intravenous injection for people who can’t absorb iron properly.
The side effects of iron supplements include nausea, vomiting, abdominal cramps, constipation, diarrhoea and anaphylactic reaction to parenteral iron.
Iron absorption to the circulation is regulated but recall that iron is readily transported into the intestinal mucosal cells without regulation. If an overdose of oral iron is taken will these mucosal cells be overloaded with iron, which is toxic for them. This causes GI bleeding or necrotizing gastritis.
The iron chelators deferoxamine (IV) and deferiprone (oral) are used to treat iron overdose and haemochromatosis.
Vitamin B12
We know by now that vitamin B12 and folic acid are also necessary for RBC production. Vitamin B12 exists in two forms that can be given orally or intramuscularly: hydroxocobalamin and cyanocobalamin. It’s found in meat, dairy products and eggs. The daily requirement is 2-3 µg.
B12 is absorbed from the terminal ileum as a complex with intrinsic factor (IF), which is secreted by parietal cells in the stomach. If the stomach’s ability to produce IF is impaired will B12 absorption also be impaired. Around 4mg of B12 is stored in the liver.
Vitamin B12 is important for DNA synthesis and for methylmalonyl-CoA mutase, which is important to produce the myelin sheath. If B12 is deficient will there not be enough DNA production to produce enough RBCs, and the myelin sheath will be deficient. It’s also needed for homocysteine metabolism – B12 deficiency causes hyperhomocysteinaemia with resulting atherogenesis and thrombogenesis.
Deficiency occurs in:
- Resection or inflammation of the terminal ileum
- Intrinsic factor deficiency – antibodies against parietal cells (pernicious anaemia) or gastrectomy
- Pregnancy
- Insufficient intake
Vitamin B12 deficiency anaemia is macrocytic and hyperchromic. This is because there is nothing wrong with the haemoglobin production but there is a problem with the production of the cell itself. Each cell will therefore contain more haemoglobin as a form of compensation. There will also be peripheral neuropathies and spinal cord degeneration due to demyelination.
Folic acid and B12 work together in DNA synthesis. In B12 deficiency can administration of folic acid compensate for the deficiency and treat the anaemia, however folic acid can not treat the demyelination. Folic acid should therefore never be used exclusively to treat B12 deficiency.
Hydroxocobalamin is the most frequently used form to treat deficiency. It’s given intramuscularly.
Folic acid
The daily recommended intake of folic acid is 200 µg per day. It’s found in liver and green vegetables. Deficiency occurs in methotrexate or phenytoin treatments and in premature babies. Folinic acid or leucovorin is a more active form of folic acid which is especially used during methotrexate therapy to reduce side-effects.
It’s well-known that pregnant women should take more folic acid, up to 400µg. This is to reduce the incidence of neural tube defects like spina bifida. What’s special about folic acid is that there is no way to overdose it – even extremely high consumption of folic acid has absolutely no side effects.
Other stuff
Pyridoxine (vitamin B6) deficiency may cause sideroblastic anaemia in susceptible individuals.
Riboflavin (vitamin B2) deficiency may cause pure red cell aplasia.
Vitamin C deficiency may decrease iron absorption as vitamin C forms complexes with iron to increase the absorption of it.
Copper deficiency may also cause microcytic hypochromic anaemia, but only in severe cases. Copper sulphate may be given.