Table of Contents
Page created on December 12, 2018. Last updated on December 18, 2024 at 16:56
Renal blood flow
The kidney receives enormous amounts of blood flow. The renal blood flow (RBF) is around 1200 mL or 20 – 25% of the cardiac output in rest. However, this large blood flow isn’t because the kidneys need a lot of oxygen (they don’t, as we’ll see later), but rather because the glomerular filtration rate depends on the RBF.
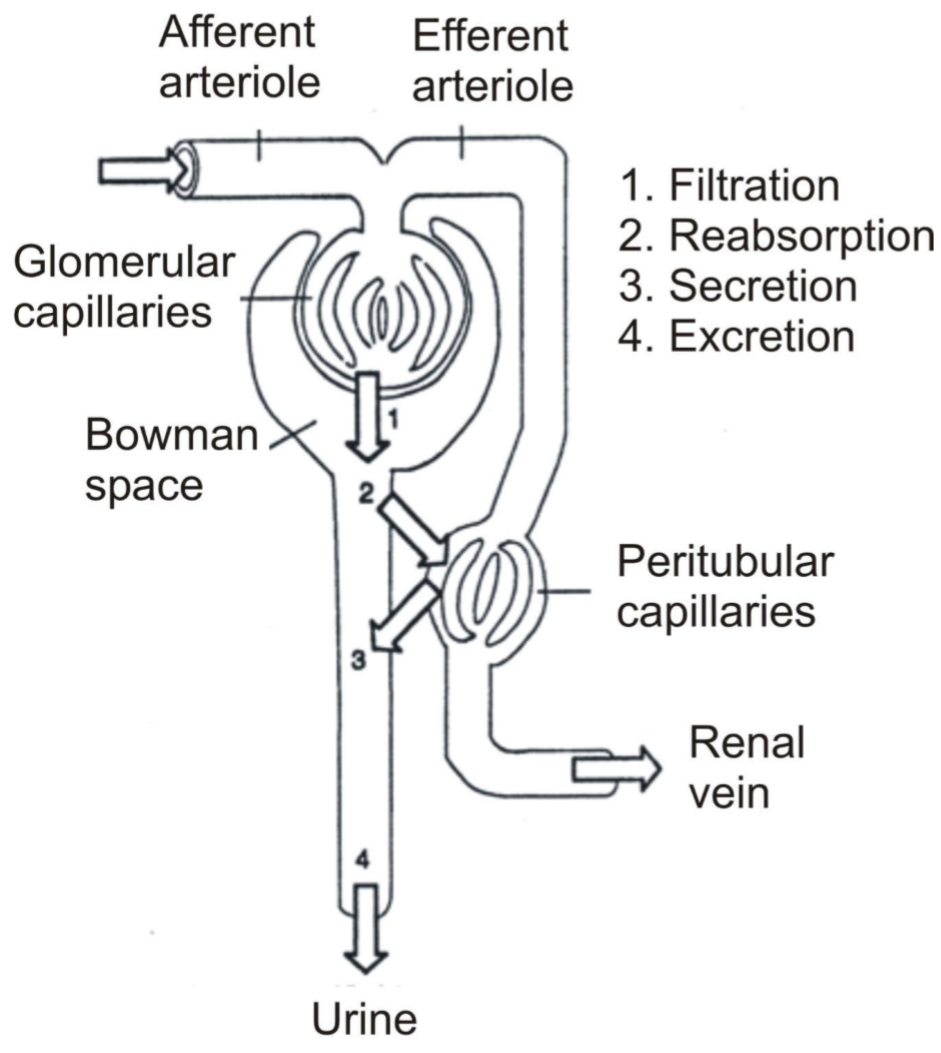
Renal arteries enter the nephron as afferent arterioles. They then become glomerular capillaries and then efferent arterioles (still arterial blood!). The vessel will then surround the tubules before draining into the renal vein. Anything the tubules reabsorb from the filtrate will enter the peritubular capillaries.
This reabsorption is often energy-dependent (and therefore oxygen-dependent), which is why the tubules is the compartment of the kidney that requires the most oxygen. During rest is the oxygen saturation difference between arterial and venous blood (ΔA-V O2) in the kidney only 1.7 – 2.1 %. In the coronaries it is around 10%, and the average of the organs in the body is 5%.
The reason the kidney can afford to have such low oxygen utilization is because it receives much more perfusion than it needs to satisfy its oxygen demand, and it receives so much blood because the glomerular filtration rate can’t be high unless the renal blood flow is high. In fact, the kidney only starts to increase its oxygen utilization above 2% when it is severely hypoperfused, when it receives around 25% of the normal RBF, or just 5% of the CO instead of 20 – 25%! Therefore will ischaemia only occur during severe hypoperfusion, and when it does will the tubules be affected first.
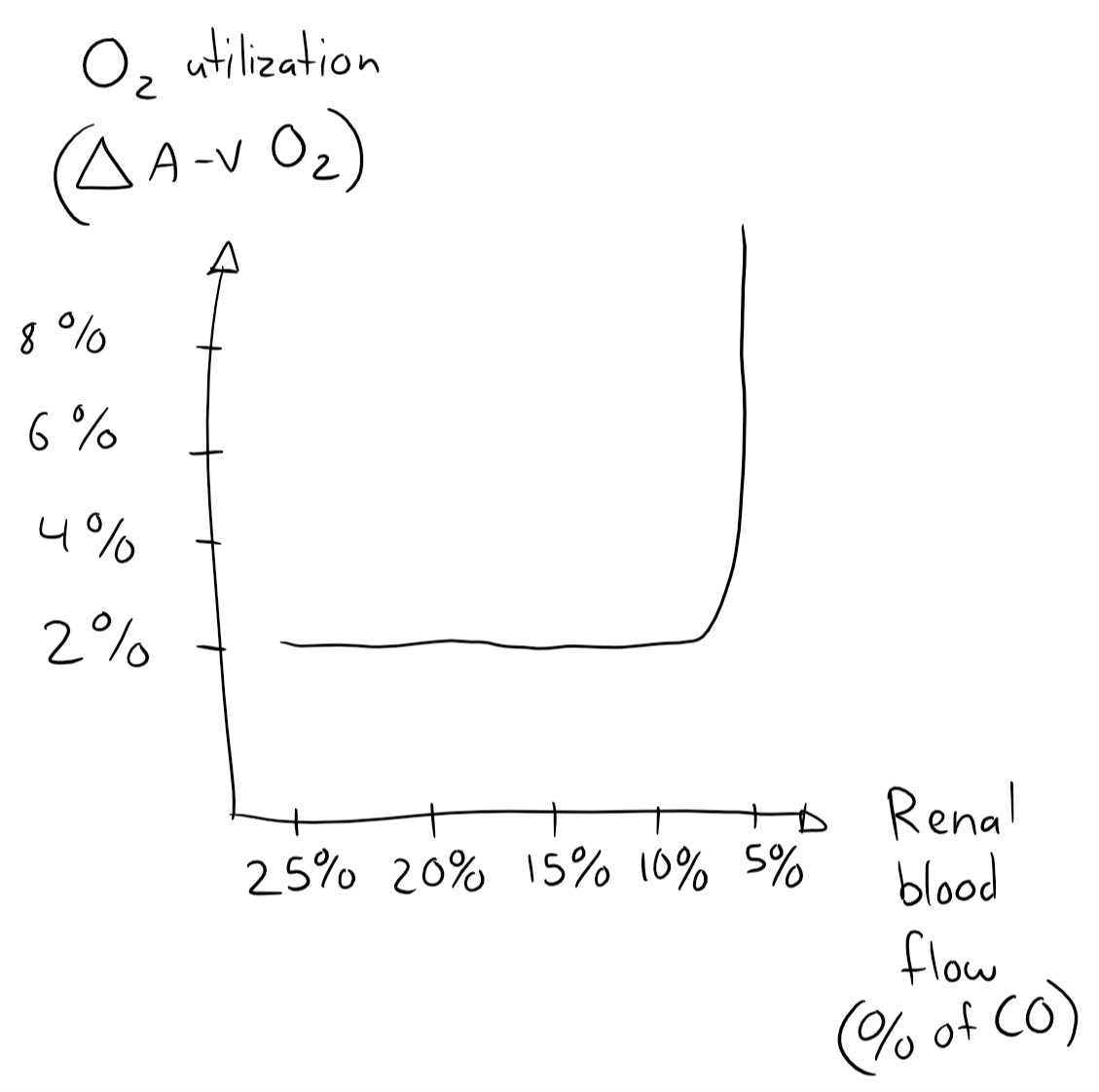
This self-made graph isn’t very important, it just illustrates that the kidney’s oxygen utilization is constant as long as it’s not severely hypoperfused.
Glomerular filtration
The normal value of the GFR is 120 – 130 mL/min, or 180 L/day. The glomerular filtration rate depends on four things:
- The renal blood flow (RBF)
- Regulated by the sympathetic tone
- The effective filtration pressure, the difference in vessel tone between the afferent and efferent arterioles
- Regulated by the tubule-glomerular feedback (TGF) system
- The size and structure of the glomerular filtration surface
- Mostly constant in physiological cases
- Important in pathological processes
- The filtration coefficient
- The permeability, pore structure and electrical charge of the filter
- Can be adjusted by humoral factors like angiotensin II, AVP
GFR can be measured by the creatinine clearance. Creatinine is 100% filtrated through the glomeruli, 0% reabsorbed and 0% secreted, so the creatinine clearance should equal the glomerular filtration rate.
The renal blood flow (RBF) is the parameter that is the easiest for the body to change in order to regulate the GFR, both physiologically and pathologically. RBF is autoregulated by vascular tone so that it is constant across a mean arterial pressure of 60 – 160 mmHg. This autoregulation is independent of innervation (it can be demonstrated in transplanted kidneys too), but the kidneys are innervated anyway. This sympathetic innervation functions to constrict the renal artery during exercise or when cardiac output must be redistributed, like during shock.
The tubule-glomerular feedback (TGF) also ensures a stable GFR. Within each nephron will information from the renal tubules be signalled to the glomerulus, which can then decide whether it needs to alter the vascular tone of the afferent and efferent vessels, depending on whether the GFR is too low or too high. The tubules detect a too high GFR by detecting how much sodium reaches the distal tubules and macula densa. If Na+ reabsorption in proximal tubules is enhanced compared to normal will the distant tubules think that the GFR is lower than normal and increase the GFR by changing the effective filtration pressure. Likewise, if Na+ reabsorption is decreased, for example in tubular damage, will the distant tubules interpret that as the GFR being too high and decrease it.
The size and structure of the glomerular filtration surface normally prevents molecules larger than 65 kD from being filtered. Changing this doesn’t just regulate how much is filtered but also what is filtered.
The filter coefficient depends on the surface area of the filtering membrane and its permeability. It can be modified by the following factors:
- Coefficient is increased by:
- Glucocorticoids
- Atrial natriuretic factor (ANF)
- Coefficient is decreased by:
- Angiotensin II
- AVP
- Prostaglandin E1
- Bradykinin
- PTH
The GFR can be calculated by the filtration coefficient x the filtration pressure. The coefficient depends on the surface area for filtration and its permeability. The filtration pressure depends on the RPF and the difference in hydrostatic pressure in the efferent and the afferent arteriole (the effective filtration pressure).
Abnormalities of the filter surface
The glomerular filtration surface is comprised of endothelium, basement membrane and the feet of podocytes. The mesangial cells don’t directly contribute to the filtration surface but support the capillary tuft structure of the glomerulus and influence the filtration by other mechanisms.
Endothelial cells produce vasoconstrictors, vasodilators and anticoagulants. Damage to the endothelial cells cause them to produce more vasoconstrictors, less vasodilators and less anticoagulants, which decreases the capillary perfusion and increases coagulation, which decreases the filtration as well. Endothelial cells are usually damaged by inflammation.
Podocytes have feet that surround the glomerular capillaries but leave small gaps between them. Anything that is to be filtered must pass through these gaps, so the podocytes can control filtration by controlling the gaps. The feet are also electronegative, making it very hard for negative proteins to be filtered.
Like endothelial cells can podocytes be damaged by inflammation, mostly due to immune-complex deposition. This causes the gaps between the feet to be larger and less negative, which may allow proteins to be filtered.
Mesangial cells originate from the bone marrow and are also phagocytes. They can contract to regulate the GFR, they produce the extracellular mesangial matrix, and they phagocytose and remove macromolecules and immune-complexes. Mesangial cells can become activated by abnormal filtrate or inflammation, which causes them to proliferate. This proliferation causes mesangial cells to produce more extracellular matrix, which will compress the rest of the glomerulus and impair its function. This process is called glomerulosclerosis and may allow proteins to be filtrated, causing proteinuria.
The basement membrane can be the target for antibodies, especially in Goodpasture syndrome. This causes the basement membrane to thicken, which paradoxically increases its permeability.
Disorders of glomerular filtration
The glomerular filtration can either be abnormal in its quantity (the amount of filtration) or its quality (what it filtrates). We must first explain a term called single nephron glomerular filtration rate (SNGFR). If the total number of nephrons decreases can the individual nephrons work harder (SNGFR ↑) to compensate.
Abnormal quality:
- Prerenal causes (problems “before” the kidneys). The blood contains haemoglobin, myoglobin, direct bilirubin, immunoglobulin chains, hyperglycaemia, which they normally shouldn’t, allowing them to be filtered.
- Renal causes (problems with the kidneys). Glomerular inflammation or tubular disorders (cause problems because of TGF)
- Postrenal causes (problems “after” the kidneys). Postrenal obstruction causes backward pressure, which decreases the filtration pressure and selectivity
Abnormal quantity:
- Hypofiltration (too low GFR)
- Exsiccosis
- Physical activity
- Shock
- Heart failure
- Toxic tubular damage (due to TGF)
- Old age
- Late phase of chronic renal failure
- Hyperfiltration (too high GFR)
- Pregnancy (increased RBF)
- Diabetes mellitus (increased glucose reabsorption in proximal tubules also causes increased Na+ reabsorption, tricking the TGF into believing that the GFR is too low)
- Protein-rich diet (protein reabsorption ↑ –> Proximal Na+ reabsorption ↑, which activates TGF)
- Early phase of chronic renal failure (to compensate for fewer nephrons)
Hyperfiltration may seem like a good compensatory mechanism to nephron loss, however hyperfiltration will actually further damage the nephrons, causing further nephron loss.
How can increased Na reabsorption indicate too low GFR? and vice versa? Shouldn’t too much Na reabsorption indicate too high GFR?
The tubules estimate the GFR by sensing the Na content of distal filtrate. When excessive Na reabsorption occurs, the content of Na in the distal filtrate is as low as it would be if the GFR is low, and so the tubules think the GFR is low.
How does thickening of basement membrane increase permeability ?
Well it is paradoxical, so I don’t know. I suspect it’s because when the basement membrane thickens the distance between the podocyte feet increases.
Hi,
Just wanted to tell you that the increase and decrease for filtration coefficient is opposite to what you have written.
AtII, vasopressin, PGE1 etc DECREASE filtrate coefficient, while cortisol and atriopeptin INCREASES it.
Right, I just checked. Fixed now. Thanks!