Table of Contents
Page created on April 8, 2018. Last updated on December 18, 2024 at 16:55
Both membrane-bound (the BCR) and secreted immunoglobulins are identical in any given B-cell, except that the membrane-bound immunoglobulin has a transmembrane and cytoplasmic part. When the B-cell wants to produce secreted immunoglobulins instead, they splice the exons that code for the transmembrane and cytoplasmic part out of the mRNA before translation. This causes the immunoglobulin to be translated without these two parts, which causes them to be secreted instead of bound to the membrane.
What is really the point of having immunoglobulins?
Before an immunoglobulin has bound an antigen, they only have one function: to bind an antigen. We say that immunoglobulins are monofunctional before they bind the antigen.
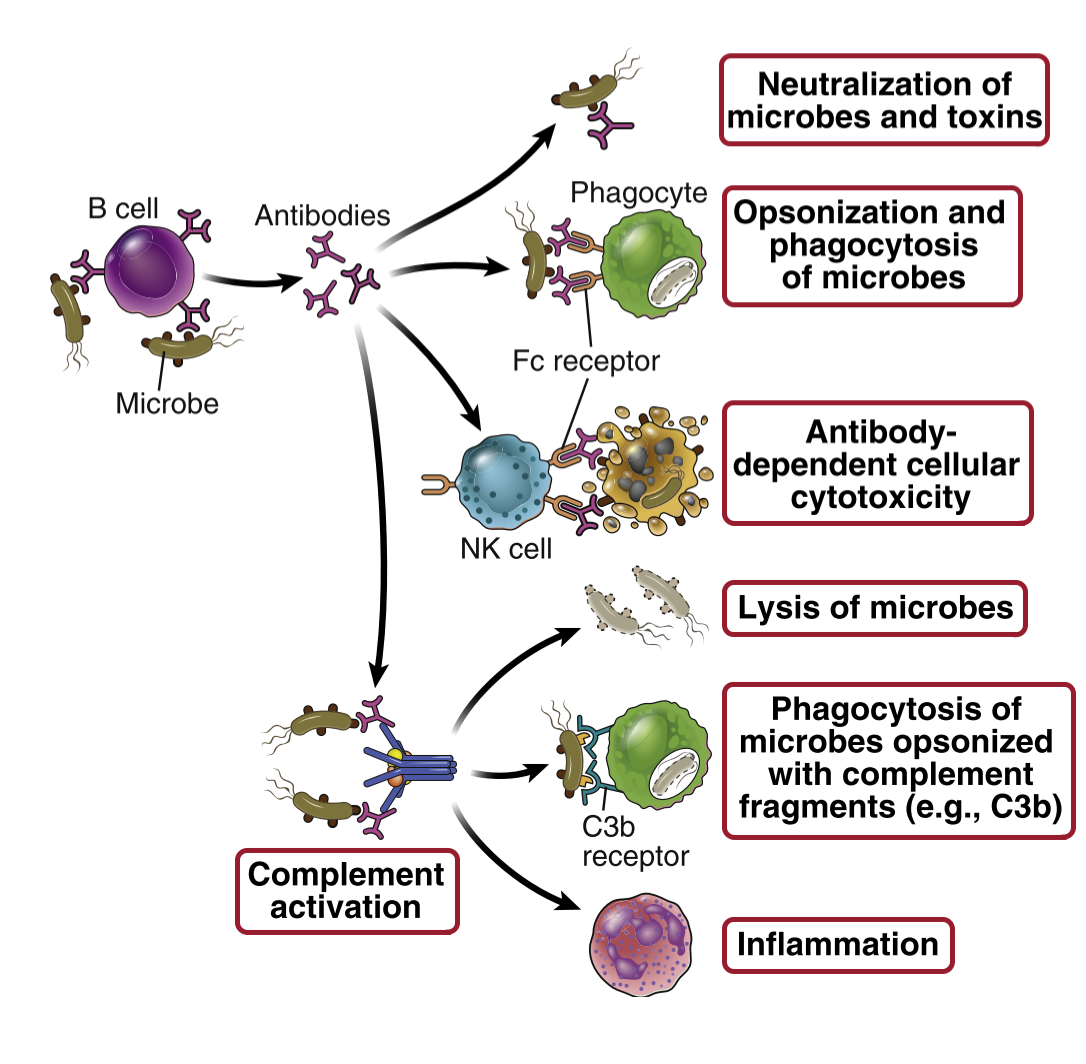
However, after an immunoglobulin has bound an antigen, it starts to have many functions. We say that they are polyfunctional after binding the antigen and forming a complex with the antigen.
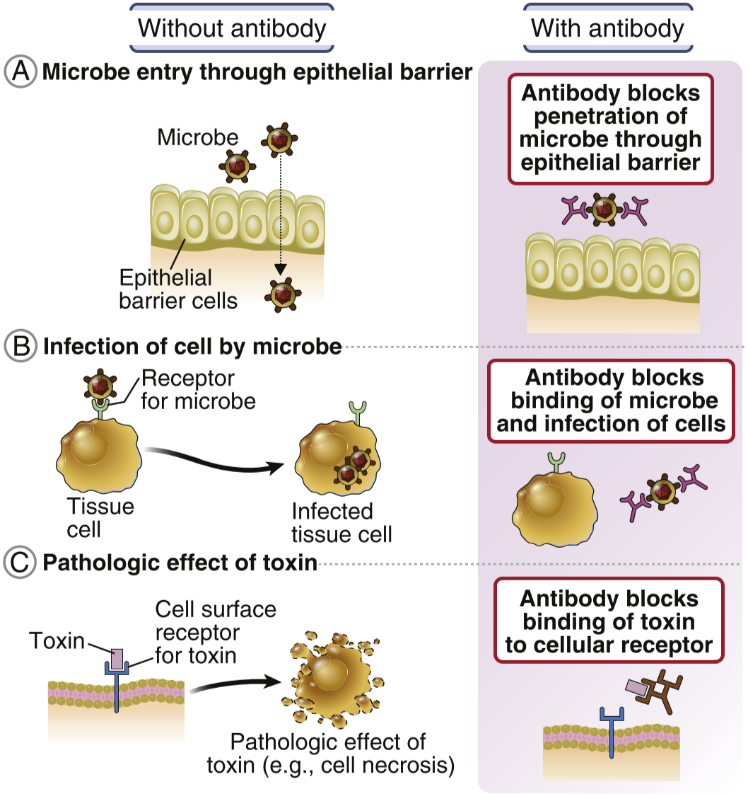
Antibodies eliminate threats in many ways. It can neutralize toxins by binding to them, so the toxins cannot do anything toxic anymore. Antibodies can also neutralize viruses. They can precipitate soluble molecules, so that they no longer are soluble and cannot be harmful to the body. They can eliminate threats by agglutination, which is simply clumping cells and particles together. Agglutination makes it impossible for pathogens to enter tissue to cause damage.
If enough antibodies bind to a bacterium (or any other pathogen), they will cover it. When a bacterium is covered in antibodies, it’s unable to do anything harmful to the body, and phagocytes have an easier time phagocyting them. This process is called opsonization. Lastly, antibodies can eliminate threats by recruiting the complement system, which we will see in detail in the next topic.
Fc receptors
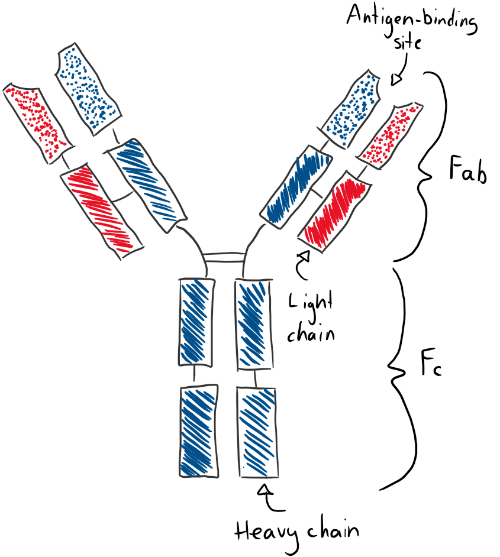
Recall that an immunoglobulin is comprised of two parts: The Fab part, which binds to the antigen, and the Fc part. Many cells have Fc receptors, that bind the Fc part of the immunoglobulin. However, the Fc receptor will only bind to an immunoglobulin that has already bound an antigen. This is because when an immunoglobulin binds an antigen, it undergoes a conformational change that changes the Fc part so that it can be bound to by Fc receptors. FcγR receptors can bind the Fc part of IgG, FcεR can bind IgE, and FcαR can bind IgA.
Different Fc receptors are found on different cell types. The FcγR comes in different types, FcγR I, FcγR II and FcγR III. FcγR II is the only one of the Fc receptors that has an inhibitory effect on the B-cell it’s attached to. FcγR III is found on NK-cells and induces ADCC, which we will read about further down.
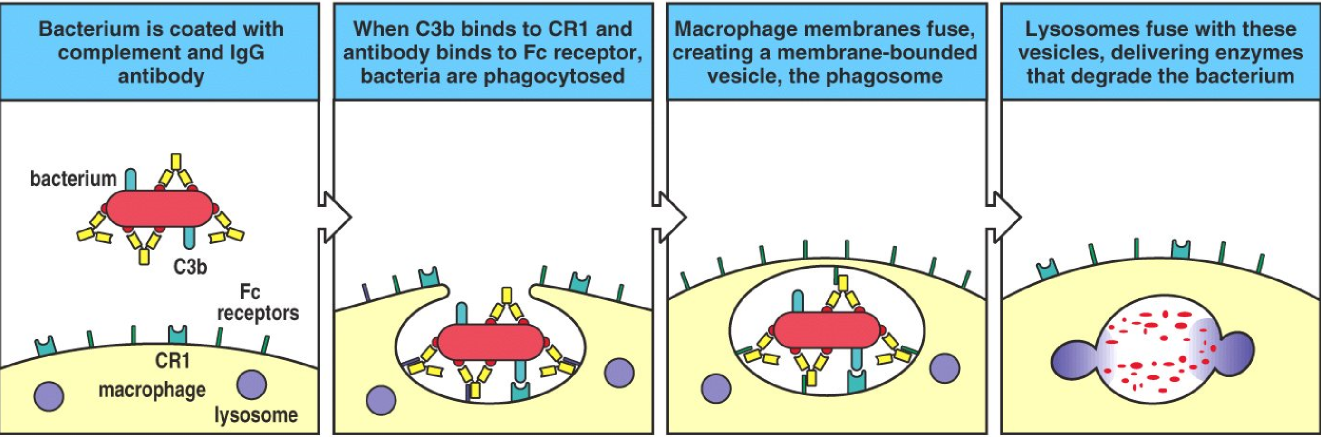
When bacteria are opsonized by IgG and complement factors the opsonized bacteria will meet a macrophage. Macrophages have both FcγR and complement receptors (that bind to the complement factors), so the macrophage can more easily phagocytose the opsonized bacteria.
The different isotypes of immunoglobulins have different properties
IgM is the immunoglobulin that is produced during the earliest response to a pathogen, see the last topic for details. IgM can cross epithelium to enter lumens of organs, like the GI tract. It is very involved in activating the complement system. Few cells have Fc receptors for IgM, so they’re not so important.
IgD is never found in free, soluble form, but only on the surface of B-cells (as their BCR).
There are four types of IgG antibodies, IgG1, IgG2, IgG3 and IgG4. They have similar properties for the most part, but certain differences. They are the most important antibodies in terms of entering tissue. IgG1 and 3 can cross the placenta in great amount, IgG2 and 4 only in small amounts. All IgG types perform neutralization and opsonization. IgG1 and 3 activate something called antibody-dependent cell-mediated cytotoxicity, which we will see more about later. All 4 types except IgG4 activate the complement system.
IgA is the most important antibody in terms of entering lumens of organs, like the GI tract. They also protect the mucosa. They do this in dimeric form. Because the GI tract contains many proteases that would degrade it, dimeric IgA contains a domain called the secretory component, which inhibits proteases. Like IgG, they can enter tissue, but in a smaller scale than IgG. They enter tissue in monomeric form. IgA neutralizes and opsonizes and activates the complement system.
IgE can enter tissue and is the only antibody that can activate mast cells. They’re involved in defense against parasites and in allergies. Also, because no rule doesn’t have exceptions, here comes an exception to the rule that only antibodies that have bound an antigen can be bound by Fc receptors. The FcεR I can bind IgE that have not yet bound an antigen. This is important in the activation of mast cells.
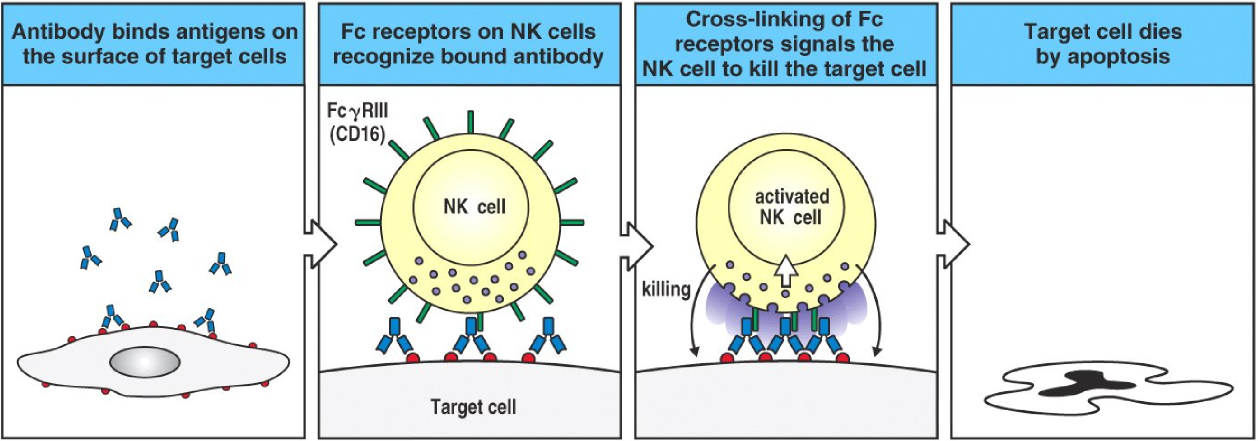
Antibody-dependent cellular cytotoxicity
ADCC, or antibody-dependent cellular cytotoxicity has been mentioned a couple of times already. It’s actually a very simple process, despite the name. It mostly applies to NK cells and eosinophils and works on eukaryotes. After an infected cell or a bacterium is opsonized by IgG, many FcγR III found on NK cells will bind to those IgG. This signals the NK cell to release killing enzymes, like perforin and lytic enzymes, which kills the target cell.
In the case of eosinophils, a parasite must be coated by IgE. Many FcεR on the eosinophil will bind to these IgE, which will cause the eosinophil to release its toxic granules to kill the parasite.