Page created on March 6, 2018. Last updated on November 19, 2018 at 17:16
While B-cells can bind free antigens in the blood and tissues, T-cell receptors can only bind to antigens that are being presented to them by other cells. This means that a T-cell cannot recognize a bacterium or virus floating past; some other cell has to digest the bacterium or virus and then present a small part of it, an antigen, from the pathogen to the T-cell for it to be activated.
A protein called major histocompatibility complex or MHC is important for this presentation. There are two types of MHC, MHC class I and MHC class II. The first type is found on all cells that have a nucleus + platelets (not RBCs). MHC II is found only on special cells called professional antigen-presenting cells (the name will make more sense soon). Dendritic cells, B cells, macrophages and monocytes belong to this category. These 4 cell types have both MHC I and MHC II on their surfaces.
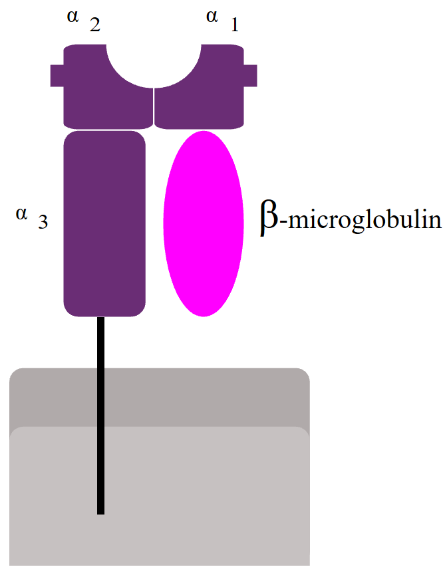
In every single cell in your body, proteins that are not needed are being broken down by a cellular machine called the proteasome. When the proteasome breaks down these proteins, short fragments of them (8-10 amino acids) are bound to an MHC I molecule and transported to the cell surface, where it will be embedded. A subtype of T-cell called cytotoxic T-cell can then recognize and bind to the MHC I – antigen complex.
Because MHC I only presents proteins that originate inside the cell, its function is to prove to immune cells that the cell is indeed from the host and not from a foreign source, and that it has not been infected. When an immune cell binds to the MHC I – antigen complex of a healthy cell, it will recognize that the short peptide presented is not foreign, so the cell won’t do anything. If a virus infects a cell however, the virus will make the cell produce viral proteins, that obviously do not belong in the body. The cell will then break down these viral proteins and present them on the MHC I. An immune cell will then recognize that this peptide is foreign and kill the cell to prevent the virus from spreading.
Many proteins are needed for this presentation. When the proteasome breaks down proteins, the short fragments are transported into the ER by a protein called TAP, the transporter associated with antigen processing. At the same time, ribosomes produce MHC I molecules and deposit them into the ER. When the MHC I molecule and the protein fragment meet, they will bind, and the MHC I – protein fragment complex will be exported to the cell surface. Several chaperones are involved in MHC I antigen presentation. They are calnexin, calreticulin, Erp57 and tapasin.
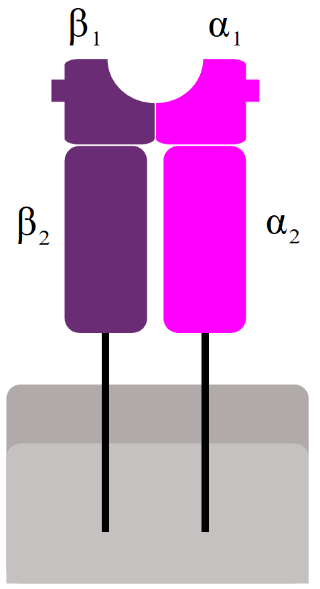
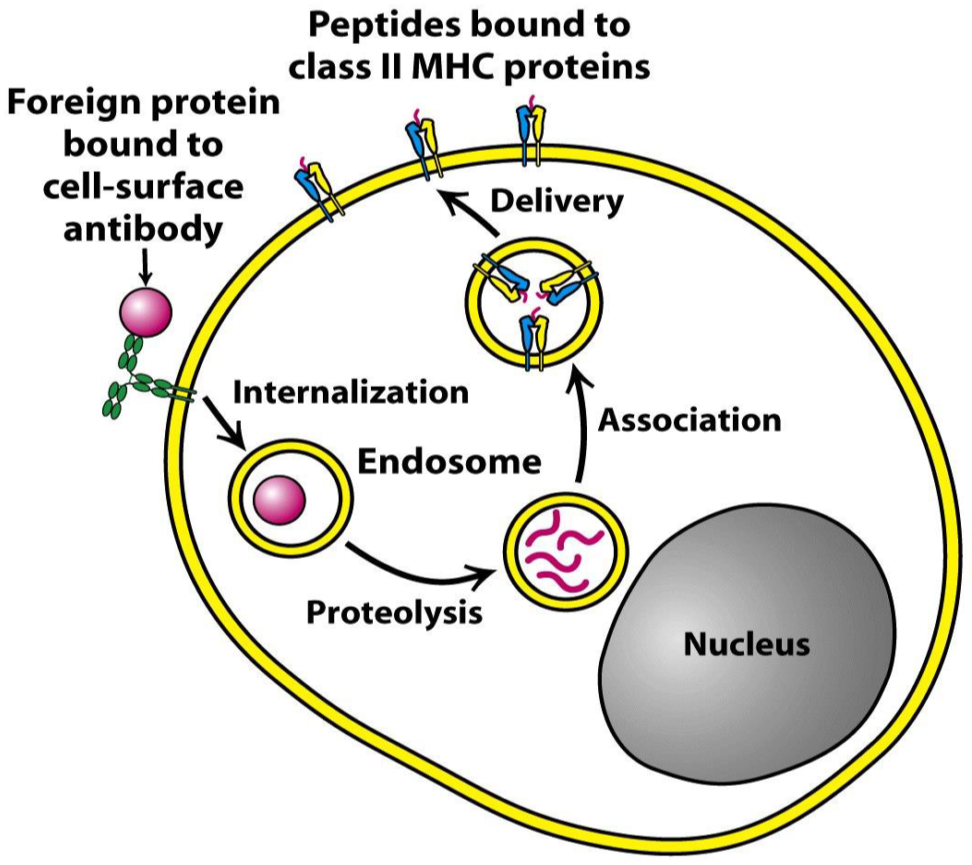
MHC II is used to present antigens in a similar way, with one big difference: The antigens it presents originate outside the cell instead of inside it; they are exogenous instead of endogenous. Antigen-presenting cells are also phagocytes, that phagocyte bacteria and viruses. After phagocyting, the “food” is broken down in endosomes inside the cell by proteases. The “food” is broken down to shorter peptides, like in the case of MHC I, and presented on the cell surface. The peptides that MHC II presents are 15 – 24 amino acids long, longer than the peptides presented by MHC I.
The mechanism is similar to that of MHC I. At the same time as the ingested material is broken down and transported to ER, MHC II is synthesized by ribosomes and deposited into ER. Inside the ER, a chaperon called HLA-DM folds MHC II into correct structure. Then, a protein called CLIP covers the antigen-binding part of MHC II, so it doesn’t bind anything on its way. The MHC II is then exported in an endosome. This endosome will fuse with the endosome containing the short peptide, which is when CLIP dissociates so that MHC II can bind to the exogenous peptide.
Three genes code for MHC I, and three other genes code for MHC II. The genes for type 1 are HLA-A, HLA-B and HLA-C, and the genes for type 2 are HLA-DP, HLA-DQ and HLA-DR. These genes are highly polymorphic, which means that there exist many variations of these genes in the population.
We distinguish two major types of T cells: The CD8+ (cytotoxic) T-cell and the CD4+ (helper) T-cell. They both have a receptor called the TCR. The TCR on CD8+ T-cells can only bind to antigens presented by MHC I, while the TCR on CD4+ can only bind to antigens presented by MHC II. This is called MHC restriction. If a cell is infected by a virus, it will present a virus antigen through MHC I, and a CD8+ T-cell will bind to this MHC I – antigen complex and kill the cell. If an antigen-presenting cell meets a bacteria, it will phagocytose it and present a bacterial antigen through MHC II. A CD4+ T-cell will bind to this MHC II – antigen complex and start producing cytokines to help the immune response.
Avoiding detection
Many viruses and bacteria have developed methods of avoiding this presentation.
Herpes simplex produces a protein which inhibits TAP, which disrupts MHC I presentation.
Adenovirus produces a protein which doesn’t allow MHC I to leave the ER.
Cytomegalovirus accelerates the degradation of MHC I on the cell surface.
HIV accumulates mutations faster than the adaptive immune system can react to.
Helicobacter pylori produce a toxin which increases the pH of the lysosome, which inhibits protease activity. This way, when the bacterium is phagocyted, it’s not broken down.
Toxic shock syndrome
Some bacteria exploit the MHC – T-cell binding to wreak havoc on the body. So-called bacterial or viral superantigens activate up to 20 000 times more T-cells than a normal infection does. This causes massive production of cytokines, which can be fatal to the host if not treated.
Summary
| Name | MHC I | MHC II |
| Found on which cells? | All nucleated cells (including the APCs) and platelets | Professional antigen-presenting cells: Dendritic cells, B cells, macrophages, monocytes |
| Recognized by which T-cells? | CD8+ | CD4+ |
| Proteins involved in presentation | Calnexin, calreticulin, Erp57, tapasin, TAP | HLA-DM, CLIP |
| Presents what? | Endogenous peptides, e. g from viruses. | Exogenous peptides, e. g from bacteria |
| Important in what infection? | Viral infection | Bacterial, parasitic infection |