Table of Contents
Page created on March 14, 2018. Last updated on December 18, 2024 at 16:55
Learning objectives
- What are the types of cell surface receptors?
- Name two G-protein coupled receptors which are important in biochemistry
- What is the function of the three different G proteins?
- Name the receptor tyrosine kinase which is important in biochemistry
- Name the two types of receptor guanylyl cyclase
- Name a hormone whose receptor is a nonreceptor tyrosine kinase
- Describe the signalling pathway of nonreceptor tyrosine kinases
- What is the prototype ligand-gated ion channel?
- Describe the signalling pathway of ligand-gated ion channels
- What are the two types of nuclear receptors, and what are their differences?
- Describe the concept of amplification and why it is important
- What is the function of AMP-dependent protein kinase (AMPK)?
- Why is an increase in AMP levels a better signal of energy depletion than a decrease in ATP levels?
- What are the targets of AMPK, and which pathways are regulated?
- What is the function of mTOR?
- What is the function of scaffold proteins, and which scaffold protein is the most studied?
- Describe the signalling pathway of protein kinase C
- Describe how cells sense the oxygen level
- What is ChREBP, and what activates it?
- Which enzymes are stimulated by ChREBP?
- What are 14-3-3 proteins?
- What is NF-κB?
Cell surface receptors
Those hormones which cannot diffuse freely across the cell membrane must bind to proteins which are located on the outside of the cell membrane, so-called cell surface receptors. These cell surface receptors are embedded in the cell membrane, with two important parts: one which is on the extracellular side of the cell membrane and one which is on the intracellular side of the cell membrane.
The hormone binds to the extracellular part of the receptor. When this binding happens, the conformation of the receptor changes. This triggers some change on the intracellular part of the receptor, which initiates a process inside the cell. That process will then initiate another process, and so on. This is how the signal is transmitted from outside the cell to the inside of the cell, without the hormone itself ever entering the cell.
There exists many different types of cell surface receptors:
- Metabotropic receptors
- G-protein coupled receptors
- Receptor tyrosine kinase
- Receptor guanylyl cyclase
- Non-receptor tyrosine kinases
- Ionotropic receptors
- Ligand-gated ion channels
Metabotropic receptors are those which act via a second messenger, which is explained below. Ionotropic receptors don’t act via a second messenger but rather by opening ion channels.
G-protein coupled receptors
- Adrenergic receptors
- Dopamine receptors
- Glucagon receptors
- Anterior pituitary hormone receptors
G-protein coupled receptors are the biggest class of cell surface receptors. Their name comes from the fact that they are coupled (but not bound) to a so-called G-protein on the intracellular side. The G protein is composed of three subunits, one α, one β and one γ.
- When glucagon (or any G-protein coupled receptor hormone) binds to its receptor, the receptor undergoes a conformational change.
- This change causes the intracellular part of the receptor to bind the G protein.
- This binding activates the G protein
- The activated G protein binds a GTP
- The binding of GTP causes the α subunit of the G protein to dissociate from the other two subunits
- The α subunit will then activate a different target protein, which furthers the signal transduction
There are three types of G proteins. The three different types act on different target proteins:
- Gs protein – acts on the enzyme adenylyl cyclase and stimulates it
- Gi protein – acts on the enzyme adenylyl cyclase and inhibits it
- Gq protein – acts on the enzyme phospholipase c and stimulates it
Adenylyl cyclase is an enzyme which converts ATP to cyclic AMP, often abbreviated as cAMP. Phospholipase C is an enzyme which cleaves phospholipids in the cell membrane into two molecules, IP3 and DAG.
cAMP, IP3 and DAG are so-called second messengers. They are small molecules which are synthesizes in large amounts in response to a hormone binding to a receptor, like we’ve seen. These second messengers will then stimulate other proteins, which will change the cell’s behaviour in some way.
cAMP activates a protein called protein kinase A (PKA). IP3 and DAG activate a protein called protein kinase C (PKC). More about these later.
When described stuff that occurs after the binding of the hormone to the receptor, we use the term downstream. Think of the signal transduction as a river. What occurs further down the river occurs downstream. This means that the second messengers act downstream from the receptors, and PKA and PKC act downstream from the second messengers.
Each G protein coupled receptor subtype is coupled to one of the three types of G protein. For example:
- α1-adrenergic receptor is Gq-protein coupled
- α2-adrenergic receptor is Gi-protein coupled
- β-adrenergic receptor is Gs-protein coupled
- Dopamine D2 receptor is Gi-protein coupled
- Histamine H1 receptor is Gq-protein coupled
- Histamine H2 receptor is Gs-protein coupled
Let’s continue the process from above. We left off at step 6, and our example is glucagon and the glucagon receptor, which is Gs-protein coupled.
7. The α subunit of the Gs protein will stimulate adenylyl cyclase
8. Adenylyl cyclase will convert many ATP molecules into many cAMP
9. cAMP will activate many protein kinase A molecules
10. Protein kinase A will change the behaviour of the cell. If the cell in question is a liver cell, the cell will initiate glycogenolysis and gluconeogenesis, and stop glycolysis.
Receptor tyrosine kinases
- Insulin receptor
The only important receptor tyrosine kinase (RTK) is the insulin receptor. It functions similarly to the G protein coupled receptors, except the receptor is not coupled to a G protein. Instead, when the hormone binds to the receptor, the intracellular part of the receptor phosphorylates itself (autophosphorylates). Let’s look at the whole process.
- Insulin binds to the insulin receptor
- The insulin receptor undergoes dimerization, a process where to insulin receptors “bind” together
- The two dimerized insulin receptors phosphorylate each other, which is called autophosphorylation
- The autophosphorylation causes the intracellular part of the insulin receptor to gain tyrosine kinase activity, meaning that it can phosphorylate tyrosine amino acid residues on other molecules
- The insulin receptor phosphorylates and activates many downstream targets, like PI3K, insulin-sensitive kinase (ISK), insulin receptor substrate (IRS)
- Each of the downstream target active further downstream processes which alters the behaviour of the cell
More about insulin in its topic.
Receptor guanylyl cyclase
Receptor guanylyl cyclase is special and exists in two forms; one “normal” form which is membrane bound like GPCR and receptor tyrosine kinases, and one “soluble” one, which is not bound to the cell membrane but rather floats around freely inside the cytoplasm.
Membrane-bound receptor guanylyl cyclase:
- Atrial natriuretic peptide (ANP)
- Brain natriuretic peptide (BNP)
When ANP or BNP binds to their receptor, the events will be similar to those of the insulin receptor. The only exception is that instead of the intracellular part of the receptor gaining tyrosine kinase activity, it gains guanylyl cyclase activity instead. Guanylyl cyclase converts GTP to cyclic GMP (cGMP), another second messenger. cGMP then activates the so-called protein kinase G (PKG).
Souble guanylyl cyclase:
- Nitric oxide (NO)
NO is a gas and therefore freely diffuses across cell membranes. This is how it can reach and bind to the soluble guanylyl cyclase, which floats around in the cytoplasm. After the binding guanylyl cyclase will convert GTP to cGMP and activate PKG, just like for the membrane-bound version.
Nonreceptor tyrosine kinase
- Erythropoietin (EPO) receptor
- Receptors for growth hormones
These receptors don’t have intrinsic kinase activity, hence the name. Instead the receptors are coupled to other proteins which do have kinase activity; these proteins are most commonly Janus kinases (JAKs).
After the hormone has bound to the receptor the receptor activates the Janus Kinase. The JAK then phosphorylates and activates another type of protein called STATs. The STATs are transcription factors which, when phosphorylated by JAK, will enter the nucleus and increase transcription of certain genes.
The proteins were initially called JAK because they were just a few kinases discovered amongst many, and so were called “Just Another Kinase”. When their importance was discovered they were renamed to Janus Kinase.
Ligand-gated ion channels
- Nicotinic acetylcholine receptors
- GABA-A receptors
- NMDA glutamate receptors
Ligand-gated ion channels, also called ionotropic receptors, are cell surface receptors which are also ion channels. When the hormone binds to the extracellular part of the ligand-gated ion channel, the ion channel in question opens up, allowing ions to flow into or out of the cell. The exact ion depends on the specific subtype of receptor.
The prototoype ionotropic receptor is the nicotinic acetylcholine receptor. It is one of two types of receptors which acetylcholine binds to, the other being muscarinic acetylcholine receptor. The receptor is present in many places in the body, most notably in the neuromuscular junction and in the ganglia of the autonomic nervous system.
After acetylcholine has bound to the nicotinic acetylcholine receptor, the ion channel will open. The ion channel in question is a non-selective cation channel, meaning that it allows all types of cations to flow into and out of the cell, most importantly Na+ and K+. Sodium will flow into the cell and potassium will flow out. This causes a depolarization of the cell membrane, which transmits the signal further.
Nuclear receptors
- Type I nuclear receptors
- Androgen receptor
- Oestrogen receptor
- Glucocorticoid receptor
- Mineralocorticoid receptor
- Progesterone receptor
- Type II nuclear receptors
- Retinoic acid receptor
- Thyroid hormone receptor
- Vitamin D receptor
There are two types of nuclear receptors, receptors which are located inside the nuclei. The hormones which bind to these receptors diffuse across the cell membrane and perhaps also the nuclear envelope to reach the receptor located inside the nucleus.
All nuclear receptors are actually ligand-gated transcription factors. This means that when the hormone binds to the nuclear receptor, the hormone-receptor complex will act as a transcription factor, changing DNA transcription in some way.
Type I nuclear receptors
Type I nuclear receptors are actually located in the cytoplasm. When the hormone diffuses across the cell membrane and enters the cytoplasm, it will bind to the type I nuclear receptor there. This hormone-receptor complex will create a dimer with another hormone-receptor complex. This dimer of two nuclear receptors and two hormones then passes the nuclear envelope through a nuclear pore, and then binds to DNA to begin DNA transcription.
Type II nuclear receptors
Type II nuclear receptors are found in the nucleus. They exist in a complex with a retinoid X receptor (RXR), and are bound to a corepressor, a molecule that represses its activity. After the hormone enters the nucleus through a nuclear pore, it will bind the nuclear receptor/RXR complex, which will cause the corepressor to dissociate. The complex will then bind RNA polymerase, which will begin transcription.
Amplification
Amplification is an important concept in signal transduction, and the reason that many signalling pathways are as complicated as they are is to achieve amplification. The term means how the signal transmitted from only one molecule of hormone can stimulate a very large cell response.
Let’s look at the glucagon pathway as an example. We now that glucagon catalyses glycogen breakdown indirectly. Imagine if the pathway was much shorter, and that the glucagon receptor directly stimulated glycogen phosphorylase. In this case, one molecule of glucagon would bind to its receptor. The receptor would stimulate one molecule of glycogen phosphorylase, which would catalyse the release of maybe 10 molecules of glucose. 10 molecules of glucose is nothing, so this would be a very weak response.
In reality, the glucagon receptor stimulates one molecule of adenylyl cyclase, which creates 20 molecules of cAMP. These 20 molecules of cAMP activate 10 molecules of PKA. Each of these 10 molecules of PKA activate 10 molecules of phosphorylase b kinase. Each of these 100 activated phosphorylase b kinase activate 10 molecules of glycogen phosphorylase. Each of these 1 000 molecules of glycogen phosphorylase catalyse the release of 10 molecules of glucose. The result is that one molecule of glucagon caused 10 000 molecules of glucose to be released, which is a much stronger signal.
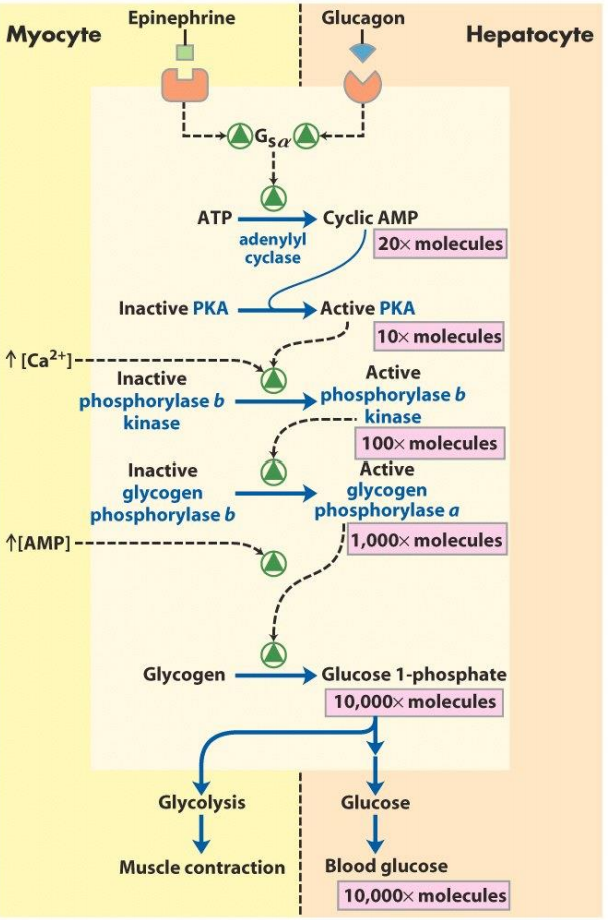
- Amplification in progress
AMPK
AMP-dependent protein kinase (AMPK) is an enzyme which is activated when cells are low in ATP. The purpose of the enzyme is to prevent complete energy depletion in the cell.
The enzyme is found in cells of many organs. The net effect of the protein is an increase in cellular uptake of glucose and fatty acids, and stimulation of metabolic processes which yield energy, like beta oxidation and glycolysis. It also inhibits processes which consume energy, like fatty acid synthesis, cholesterol synthesis, and insulin secretion.
Why is it not ATP-dependent protein kinase?
AMPK is allosterically activated by an increase in intracellular [AMP]. [AMP] is increased as the level of ATP in the cell decreases, either as a cause of exercise or not enough nutrition. AMPK is activated by increase in [AMP] rather than a decrease in [ATP] for a reason.
The cell normally contains more ATP than AMP. When a cell is “depleted” for ATP there are actually many molecules of ATP left. A normal cell has around 5,0 mM of ATP, and 0,1 mM of AMP. After being depleted of energy, the cell now has 4,5 mM of ATP and 0,6 mM of AMP. This is because 0,5 mM of ATP has been converted to AMP. The concentration of ATP has decreased by 10% from 5,0 to 4,5, but the concentration of AMP has increased by 600%. The change in concentration of AMP is much larger than the change in concentration of ATP, so the change in [AMP] is a stronger “signal” of energy depletion.
Effects
When [AMP] is high, AMPK phosphorylates many different key proteins, regulating their activity to either increase or decrease their activity, depending on the target protein. The table below shows which processes are regulated by AMPK, and what the target enzymes are:
| Pathway | Target enzyme | Activated/inhibited |
|---|---|---|
| Glycolysis (in heart) | PFK2 | Activated |
| Glucose transport | GLUT1, GLUT4 | Activated |
| Fatty acid synthesis | FAS I, ACC | Inhibited |
| Lipolysis | HSL | Inhibited |
| Cholesterol synthesis | HMG-CoA reductase | Inhibited |
| Triacylglycerol synthesis | GPAT | Inhibited |
| Glycogen synthesis | GS | Inhibited |
| Protein synthesis | eEF2, mTOR | Inhibited |
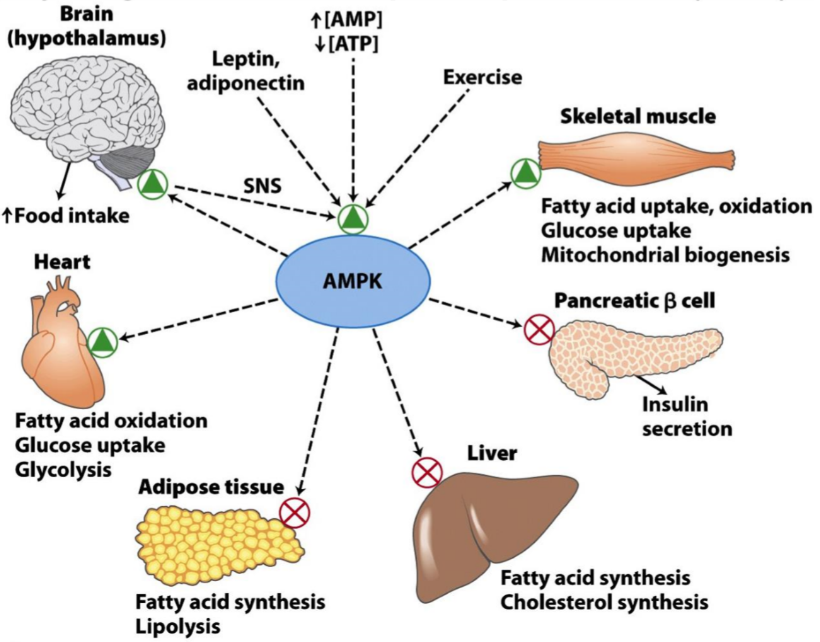
How AMP dependent protein kinase regulates different metabolic processes.
AMPK also affects cognitive functions. It will stimulate feeding behaviour, and inhibit behaviour that uses unnecessary energy. By changing the body’s behaviour to stimulate feeding and stimulate processes which yields energy from energy stores (like beta oxidation), AMPK works to increase the concentration of ATP in the cells of the body.
mTOR and mTORC1
mTORC1 (mTOR complex 1) is a protein complex comprised of mTOR and some other proteins. mTOR is the core component of this complex, and it is the component with the kinase activity which allows the complex to regulate cell processes. mTORC1 functions as a sensor for nutrients and uses this information to regulate cell growth.
Note that the terms mTOR and mTORC1 are used sometimes interchangeably, even though they’re not the same thing.
Activation
mTORC1 is activated by high concentration of nutrients and by growth factors. One of these growth factors is insulin, which activates mTORC1 through the PKB pathway, as we’ll see in the insulin topic.
Function
When activated it stimulates many processes:
- Protein synthesis
- By inhibiting 4E-BP, which inhibits eIF4E
- Ribosome biogenesis
- Glucose uptake
- The pentose phosphate pathway
- To ensure ample supply of NADPH and nucleotides
- Angiogenesis
- By activating HIF-1α
- Mitochondrial metabolism
- By activating PGC-1a
- Adipogenesis
- By activating PPAR
The sum of these processes allow for cell growth and proliferation.
Clinical relevance
mTORC1 has been widely studied in relation to aging. Overactivation of mTORC1 leads to earlier aging, and inhibition of mTOR increases the lifespan of mice. It is hypothesized that caloric restriction, which reduces the availability of nutrients, could extend the lifespan of humans by decreasing the activation of mTORC1.
mTORC1 is also relevant in cancer development. Many cancers have mutation in a gene called PTEN, which normally inhibits mTORC1.
Scaffold proteins
A situation where one protein phosphorylates another, which phosphorylates another, which phosphorylates another and so on, is common in signal transduction pathways. This is called a protein kinase cascade. As we’ll see in the insulin topic, a protein called Raf-1 phosphorylates a second protein called MEK, which phosphorylates a third protein called ERK.
To speed up this process scaffold proteins can bind all three of these proteins, thereby increasing their proximity to each other and decreasing the time it takes for them to phosphorylate one another.
These scaffolding proteins can themselves be regulated, thereby regulating the activity of the protein kinase cascade.
The best studied scaffolding protein is called KSR. It binds Raf-1, MEK, and ERK and decreases the time it takes for them to phosphorylate one another.
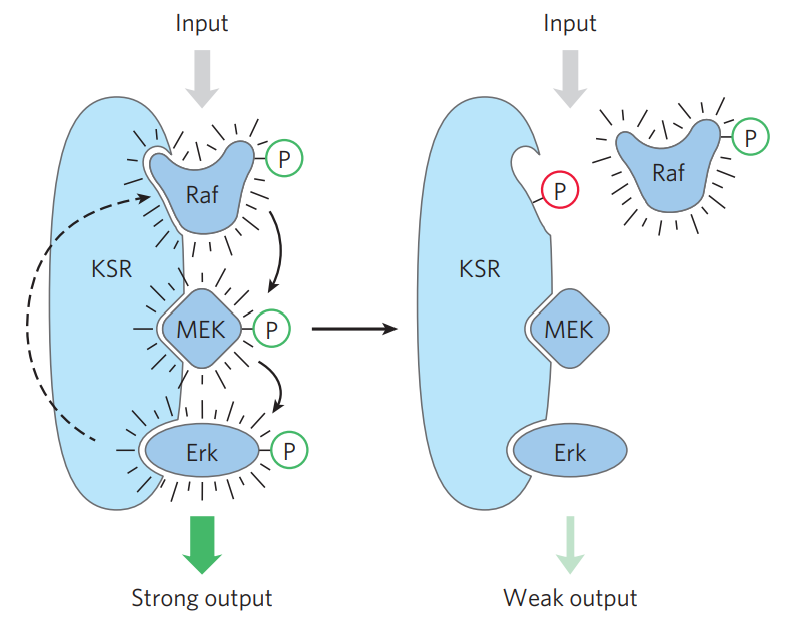
On the left: KSR is activated, increasing the activity of the protein kinase cascade. On the right: KSR is phosphorylated at the binding site for Raf, thereby preventing Raf from binding, decreasing the activity of the protein kinase cascade.
Protein kinase C
Protein kinase C (PKC) is a kinase which is involved in many different processes. It is activated by Ca2+ and diacylglycerols (DAG). It’s involved in the signalling pathway of many hormones, like oxytocin, angiotensin, histamine, vasopressin, etc.
When these hormone bind to their receptor, phospholipase C is activated. This enzyme cleaves phospholipids into IP3 and DAG. IP3 binds to a receptor on the ER, causing Ca2+ to be released from the ER. The released Ca2+ and the DAG activate protein kinase C.
When activated, PKC is involved in many functions, like learning, memory, regulating cell growth and mediating immune responses.
HIF-1α and oxygen sensing in humans
Hypoxia-inducible factor 1α (HIF-1α) is a protein that is used to sense the concentration of O2 in the cells. It is activated when cells are low on oxygen (hypoxia), and, when activated, it activates processes that yield energy without needing oxygen, while inhibiting processes that do need oxygen. It also activates processes like angiogenesis, which is important in hypoxia.
During normoxia (normal oxygen level)
HIF-1α is constantly hydroxylated at a proline residue by O2, a process which requires vitamin C and Fe2+. After hydroxylation, it binds to a protein called Von Hippel–Lindau protein, or pVHL. The HIF-1α-pVHL complex is then ubiquitinated and broken down.
During hypoxia
When O2-saturation is low, HIF-1α can’t be hydroxylated, because hydroxylation requires O2. When not hydroxylated HIF-1α acts as a transcription factor. Now that HIF-1α isn’t hydroxylated and broken down it is instead phosphorylated, which prevents it from being broken down, allowing it to do its job as a transcription factor. It will then travel into the nucleus and change gene expression of certain genes.
HIF-1α increases expression of genes that are involved in glycolysis, and lactate dehydrogenase. Because glycolysis doesn’t need O2, but the TCA and oxidative phosphorylation do, glycolysis is activated while the latter two are inhibited. It also inhibits pyruvate dehydrogenase complex.
HIF-1α also modifies complex IV of the oxidative phosphorylation. It switches out one of its subunits (COX4-1) with another subunit (COX4-2). COX4-2 is is more effective than COX4-1 when O2 is low.
The mechanism of how cells use VHL and HIF-1α to sense the oxygen level was the subject for the nobel prize of medicine and physiology in 2019. Cool stuff!
ChREBP
Carbohydrate response element binding protein, or ChREBP, is a transcription factor whose purpose is to stimulate fatty acid and lipid synthesis when the body has enough energy.
It is activated by PP2A, which is activated by xylulose 5-phosphate, a product of the pentose phosphate pathway. It is also activated by insulin.
ChREBP increases expression of L-type pyruvate kinase, acetyl-CoA carboxylase and fatty acid synthase in the liver, adipose tissue and the kidney. This increases fatty acid and lipid synthesis.
FOXO
Forkhead box other, or FOXO, is a class of transcription factors. The most important of them is FOXO1.
FOXO1 increases transcription of the gluconeogenetic enzymes PEPCK and glucose 6-phosphatase. Because insulin causes degradation of FOXO1 it indirectly decreases transcription of these genes.
14-3-3 proteins
14-3-3 proteins are a family of proteins in eukaryocytes. These proteins bind certain phosphoproteins and stabilize them, the most relevant for us being Raf, BAD, and FOXO proteins.
NF-κB
Nuclear factor kappa B, or NF-κB, is a transcription factor that is involved in cell damage and inflammation. It is activated by several stimuli, like stress, free radicals, cytokines, infection, and UV radiation.
It responds to these stimuli by increasing expression of genes related to immune response and inflammation, amongst others. It is a very central component of immunology and pathology and you’re going to hear more about it later.
Summary
- What are the types of cell surface receptors?
- Metabotropic receptors
- G-protein coupled receptors
- Receptor tyrosine kinase
- Receptor guanylyl cyclase
- Non-receptor tyrosine kinases
- Ionotropic receptors
- Ligand-gated ion channels
- Metabotropic receptors
- Name two G-protein coupled receptors which are important in biochemistry
- Adrenergic receptors
- Glucagon receptors
- What is the function of the three different G proteins?
- Gs protein activates adenylyl cyclase
- Gi protein inhibits adenylyl cyclase
- Gq protein activates phospholipase C
- Name the receptor tyrosine kinase which is important in biochemistry
- The insulin receptor
- Name the two types of receptor guanylyl cyclase
- Membrane-bound receptor guanylyl cyclase
- Soluble guanylyl cyclase
- Name a hormone whose receptor is a nonreceptor tyrosine kinase
- Erythropoietin
- Describe the signalling pathway of nonreceptor tyrosine kinases
- Nonreceptor tyrosine kinases activate JAK, which phosphorylate and activate STAT
- STATs are transcription factors which will enter the nucleus after being phosphorylated
- What is the prototype ligand-gated ion channel?
- The nicotinic acetylcholine receptor
- Describe the signalling pathway of ligand-gated ion channels
- After the hormone binds to the receptor the ion channel opens up, causing influx or outflux of ions
- What are the two types of nuclear receptors, and what are their differences?
- Type I and type II nuclear receptors
- Type I reside in the cytosol while type II reside in the nucleus
- Describe the concept of amplification and why it is important
- Amplification refers to how only one molecule of hormone can stimulate a very large cell response
- Signal transduction pathways include many steps, and at each step the signal is amplified
- As a result of amplification 1 molecule of glucagon can release 10 000 molecules of glucose
- What is the function of AMP-dependent protein kinase (AMPK)?
- AMPK is activated in energy-depleted cells. It stimulates processes which provide energy and inhibits processes which consume energy
- Why is an increase in AMP levels a better signal of energy depletion than a decrease in ATP levels?
- Because during energy depletion the change in AMP levels is much greater than the change in ATP levels
- What are the targets of AMPK, and which pathways are regulated?
- Activated
- PFK2 (in heart) – glycolysis
- GLUT1, GLUT4 – glucose intake
- Inhibited
- FAS I, ACC – fatty acid synthesis
- HSL – lipolysis
- HMG-CoA reductase – cholesterol synthesis
- GPAT – triacylglycerol synthesis
- GS – glycogen synthesis
- eEF2, mTOR – protein synthesis
- Activated
- What is the function of mTOR?
- mTOR is a kinase which, as part of the protein complex mTORC1, stimulates cell growth in response to high levels of nutrients
- It activates protein synthesis by inhibiting 4E-BP
- What is the function of scaffold proteins, and which scaffold protein is the most studied?
- Scaffold proteins bind protein kinases in a cascade, so that they lie in close proximity
- The most important one is KSR
- Describe the signalling pathway of protein kinase C
- The receptor activates phospholipase C, which cleaves membrane phospholipids into IP3 and DAG
- IP3 causes Ca2+ release from ER
- DAG and Ca2+ activate protein kinase C
- Describe how cells sense the oxygen level
- In normoxia HIF-1α is hydroxylated (with the help of vitamin C), bound to pVHL and degraded
- In hypoxia HIF-1α is phosphorylated, causing it to enter the nucleus and act as a transcription factor
- It increases expression of genes involved in glycolysis + lactate dehydrogenase
- It switches out one of the subunits of complex IV with one that is more oxygen efficient
- What is ChREBP, and what activates it?
- ChREBP is a transcription factor which stimulates fatty acid and lipid synthesis
- It is activated by PP2A, which is activated by xylulose 5-phosphate and insulin
- Which enzymes are stimulated by ChREBP?
- L-type pyruvate kinase
- Acetyl-CoA carboxylase
- Fatty acid synthase
- What are 14-3-3 proteins?
- 14-3-3 proteins bind to certain phosphoproteins and stabilize them
- What is NF-κB?
- NF-κB is a transcription factor which is activated in stress, infection, radiation, etc.
- It increases transcription of genes involved in inflammation and immune response