Table of Contents
Page created on March 14, 2018. Last updated on December 18, 2024 at 16:55
Learning objectives
- What is the purpose of insulin?
- Describe the synthesis of insulin
- Describe the process by which insulin is released in response to high blood glucose
- Which biochemical processes are simulated by insulin?
- Which biochemical processes are inhibited by insulin?
- Describe the mechanism by which insulin inhibits the transcription of two gluconeogenetic enzymes
- Describe the mechanism by which insulin stimulates glycogen synthase
- Describe the mechanism by which insulin inhibits glycogen phosphorylase
- Describe the mechanism by which insulin increases glucose uptake into cells
- Describe the pathway by which insulin activates PP1 and PP2A
- What are the functions of PP1?
- What are the functions of PP2A?
- Describe the antiapoptotic effects of insulin
- Describe the effect of insulin on protein synthesis
Insulin
Insulin is the only hormone which decreases blood glucose levels. Its purpose is to stimulate the body to store glucose as fat and glycogen, and to stimulate the synthesis of proteins and division of cells, but only when the level of glucose in the blood is high.
Synthesis
Insulin is synthesised by beta-cells in the Langerhans islets in the pancreas. It’s a peptide hormone, synthesised from preproinsulin to proinsulin to mature insulin by proteolytic cleavage.
During this cleavage a segment of proinsulin called C peptide is cleaved off. This peptide has medical relevance in that it is anti-inflammatory, and that it can be used to identify death by exogenous insulin poisoning. If someone is poisoned by exogenous insulin, they will have much lower C peptide levels than the levels of insulin in the blood would indicate.
Insulin, like most hormones, is synthesised ahead of time and stored inside vesicles, ready to be released when the signal arrives.
Stimulation of release
Insulin is synthesized by β-cells in the Langerhans islets. While GLUT4 transporters only take in glucose when insulin is present in the blood, the β-cell contains GLUT2 transporters, which only take in glucose when blood glucose is high (due to its low affinity for glucose).
This glucose is then phosphorylated by glucokinase, and the glucose is degraded to CO2 and water by glycolysis and oxidative phosphorylation like usual. The β-cell uses glucokinase instead of hexokinase, because the product, glucose-6-phosphate, inhibits hexokinase, but not glucokinase. More about the difference between hexokinase and glucokinase here.
When blood glucose is high, more glucose will be broken down in the β-cell, so the concentration of ATP in the β-cell will increase. When a certain level of intracellular ATP is reached the ATP-gated K+ channel on the cell surface will close.
When this channel closes the cell depolarizes, which opens another channel on the cell surface, the voltage-dependent Ca2+ channel. This will cause Ca2+ to enter the cell, while Ca2+ is also released from ER. The increased concentration of Ca2+ in the cell stimulates the release of insulin into the blood.
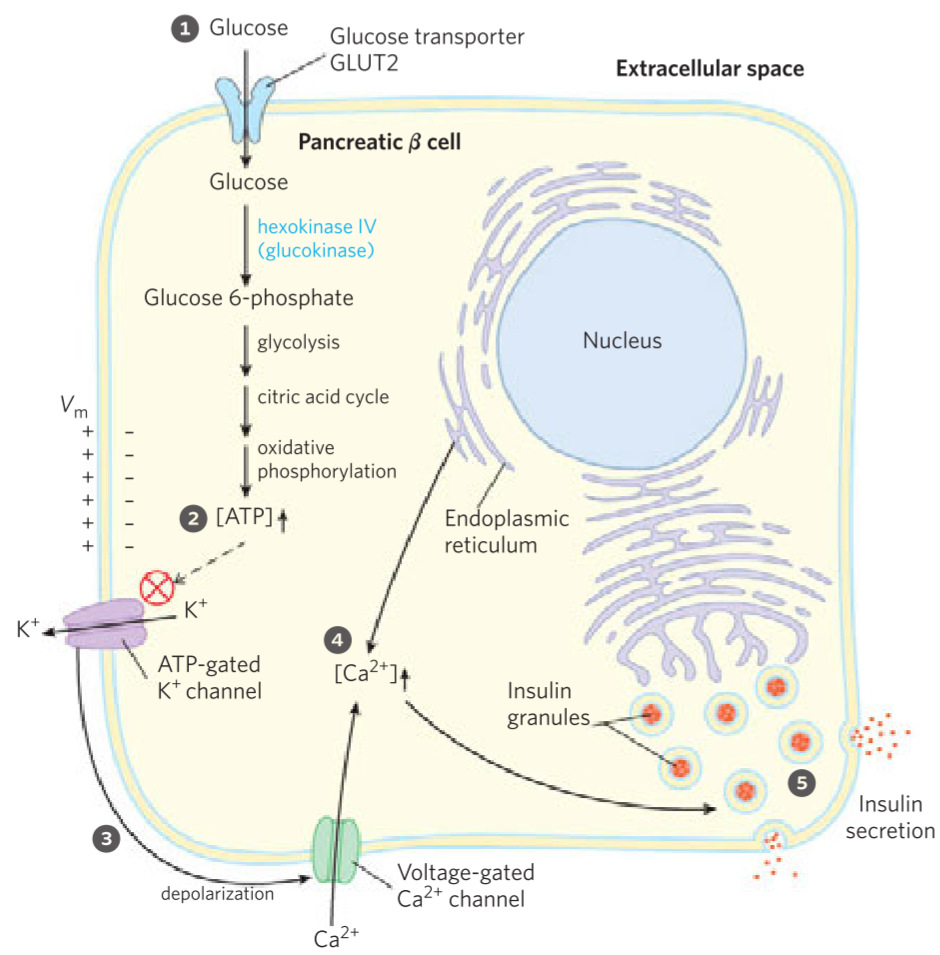
- How the β-cell releases insulin
Biochemical function
Insulin is produced in response to high blood glucose level, and it stimulates processes which reduce the blood glucose level. These processes include:
- Glycogen synthesis
- Fatty acid synthesis
- Lipid synthesis
- Protein synthesis
- Cholesterol synthesis
- Uptake of glucose into the cell, via the GLUT4 transport proteins
It also inhibits processes which increase the blood glucose level, like:
- Lipolysis
- Beta oxidation
- Proteolysis
- Glycogenolysis
- Gluconeogenesis
Right after a meal, when the GI tract has absorbed the carbohydrates from the meal, the level of glucose in the blood is high. Some of this glucose should be utilized for new cells and other anabolic processes, and the rest should be stored as glycogen or fat. Insulin mediates all these responses.
The insulin pathway
The signal transduction of insulin is described in more detail in topic 34.
The insulin pathway is complicated because it branches out, and it involves many proteins. The hormone acts both by covalently modifying enzymes and by altering gene expression, both of which are accomplished by multiple pathways.
Altering gene expression by the MEK-ERK pathway
When insulin binds to its receptor the receptor phosphorylates a protein called insulin receptor substrate 1, or IRS-1. IRS-1 activates Grb2, which activates Ras, which activates Raf-1. Raf-1 phosphorylates MEK, which phosphorylates ERK.
The phosphorylated ERK enters the nucleus and phosphorylates Elk1 and SRF. These two proteins are transcription factors which will increase transcription of genes needed for cell division.
Altering gene expression by the PKB pathway
When insulin binds to its receptor the receptor will activate protein kinase B (PKB). PKB has many functions, one of which is to phosphorylate FOXO1. When phosphorylated FOXO1 will later be ubiquitinated and degraded.
FOXO1 is a transcription factor which increases transcription of gluconeogenetic enzymes PEPCK and glucose 6-phosphatase. Because insulin causes degradation of FOXO1 it indirectly decreases transcription of these genes.
Stimulating glycogen synthesis by the PKB pathway
PKB phosphorylates and inhibits GSK3, the enzyme which inhibits glycogen synthase. By inhibiting the inhibitor of glycogen synthase insulin indirectly activates glycogen synthesis.
Stimulating glucose uptake of cells by the PKB pathway
PKB phosphorylates and inhibits AS160. AS160 is a protein which inhibits another protein called Rab.
Rab is a protein involved in moving the GLUT4 glucose transporter from intracellular vesicles to the cell membrane. Because PKB inhibits that which inhibits GLUT4 translocation to the cell membrane, it indirectly stimulates GLUT4 translocation.
Translocation of GLUT4 to the cell membrane increases the amount of glucose these cells can take in from the blood. This occurs mostly in adipocytes and skeletal muscle cells.
The ISK pathway
After insulin binds to its receptor the receptor will activate insulin-sensitive kinase (ISK). ISK activates two proteins, PP1 and PP2A. Both of these are protein phosphatases, meaning that they dephosphorylate proteins.
PP1 alter the functions of many enzymes, so they’re listed in a table below.
| Enzymes affected by PP1 | ||
| Glycogen synthase | Activated | Glycogen synthesis |
| Glycogen phosphorylase | Inactivated | Glycogen breakdown |
| Glycogen phosphorylase kinase | Inactivated | Activates glycogen phosphorylase |
| Fructose 2,6-bisphosphatase | Inactivated | Decreases glycolysis |
| Phosphofructokinase-2 | Activated | Increases glycolysis |
| Pyruvate kinase (liver) | Activated | Glycolysis |
PP2A stimulates HMG-CoA reductase and acetyl-CoA carboxylase, thereby increasing cholesterol and fatty acid synthesis, respectively. PP2A also activates the ChREBP transcription factor, which increases the transcription of many enzymes, including:
- L-type pyruvate kinase
- Acetyl-CoA carboxylase
- Fatty acid synthase
Antiapoptotic effects
Apoptosis is programmed cell death. Insulin inhibits apoptosis through the effects of PKB. PKB inhibits the pro-apoptotic protein called BAD.
Effects on protein synthesis
PKB stimulates mTOR. mTOR inhibits 4E-BP, the protein which inhibits eIF4E, the eukaryotic initiation factor 4E. Thus, insulin stimulates protein synthesis.
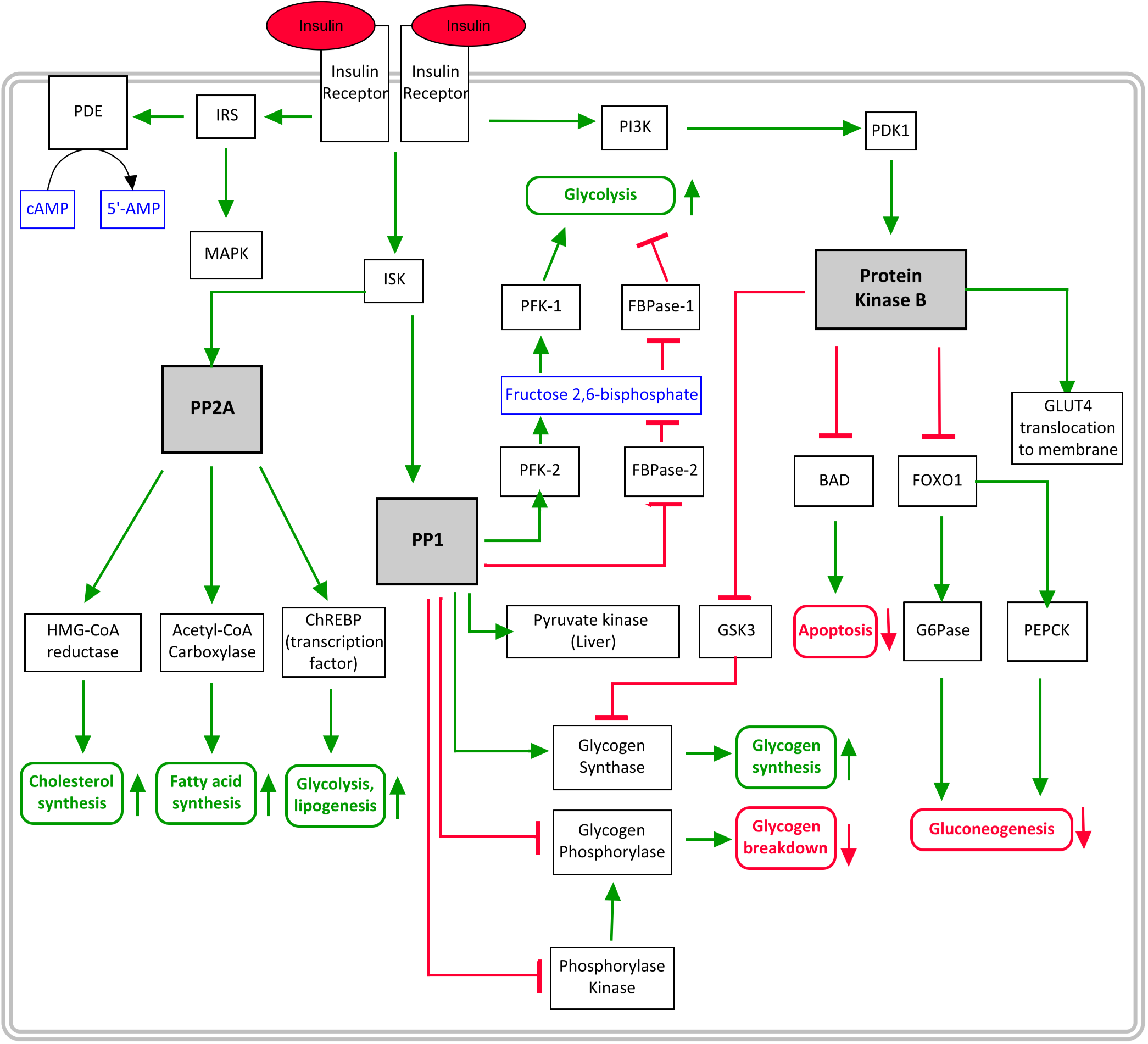
- Some of the various pathways which are activated by insulin
Summary
- What is the purpose of insulin?
- Insulin decreases the blood glucose by stimulating cells to take in glucose and use it to synthetise glycogen, fat, cholesterol, and proteins
- Describe the synthesis of insulin
- It is synthesised by beta-cells in the Langerhans islets in the pancreas
- It is synthesised from preproinsulin to proinsulin to mature insulin by proteolytic cleavage
- Describe the process by which insulin is released in response to high blood glucose
- β cells take in glucose through the GLUT2 transporter, and then phosphorylate it by glucokinase
- G6P then enters the glycolysis and oxidative phosphorylation to yield ATP
- When the blood glucose level reaches a certain level the level of intracellular ATP will cause the ATP-gated K+ channel on the cell surface to close. This depolarizes the cell
- The depolarization causes the voltage-dependent Ca2+ channel to open, causing calcium influx into the cell
- The increased intracellular calcium stimulates release of stored insulin into the blood
- Which biochemical processes are simulated by insulin?
- Glycogen synthesis
- Fatty acid synthesis
- Lipid synthesis
- Protein synthesis
- Cholesterol synthesis
- Glucose uptake by cells through GLUT4 proteins
- Which biochemical processes are inhibited by insulin?
- Lipolysis
- Beta oxidation
- Proteolysis
- Glycogenolysis
- Gluconeogenesis
- Describe the mechanism by which insulin inhibits the transcription of two gluconeogenetic enzymes
- Insulin -> insulin receptor -> PKB
- PKB phosphorylates and degrades FOXO1
- FOXO1 is a transcription factor which, when not degraded, increases the transcription of PEPCK and glucose 6-phosphatase
- Describe the mechanism by which insulin stimulates glycogen synthase
- Insulin -> insulin receptor -> PKB
- PKB phosphorylates and inhibits GSK3
- GSK3, when uninhibited, inhibits glycogen synthase
- Describe the mechanism by which insulin inhibits glycogen phosphorylase
- Insulin -> insulin receptor -> ISK -> PP1
- PP1 inhibits glycogen phosphorylase directly
- PP1 also inhibits glycogen phosphorylase indirectly, by inhibiting glycogen phosphorylase kinase
- Describe the mechanism by which insulin increases glucose uptake into cells
- Insulin -> insulin receptor -> PKB
- PKB phosphorylates and inhibits AS160
- AS160, when uninhibited, inhibits Rab
- Rab moves GLUT4 glucose transporter from intracellular vesicles to the cell membrane
- Describe the pathway by which insulin activates PP1 and PP2A
- Insulin -> ISK -> PP1 and PP2A
- What are the functions of PP1?
- PP1 stimulates
- Glycogen synthase
- Phosphofructokinase 2
- L-type Pyruvate kinase (in liver)
- PP1 inhibits
- Glycogen phosphorylase
- Glycogen phosphorylase kinsae
- Fructose 2,6-bisphosphatase
- PP1 stimulates
- What are the functions of PP2A?
- PP2A activates
- HMG-CoA reductase
- Acetyl-CoA carboxylase
- The ChREBP transcription factor, which increases transcription of
- L-type pyruvate kinase
- Acetyl-CoA carboxylase
- Fatty acid synthase
- PP2A activates
- Describe the antiapoptotic effects of insulin
- Insulin -> insulin receptor -> PKB
- PKB phosphorylates and inhibits the pro-apoptotic protein BAD
- Describe the effect of insulin on protein synthesis
- Insulin -> insulin receptor -> PKB
- PKB stimulates mTOR
- mTOR inhibits 4E-BP
- 4E-BP, when uninhibited, inhibits eIF4E, one of the major regulating points of protein synthesis