Table of Contents
Page created on May 3, 2018. Last updated on December 18, 2024 at 16:56
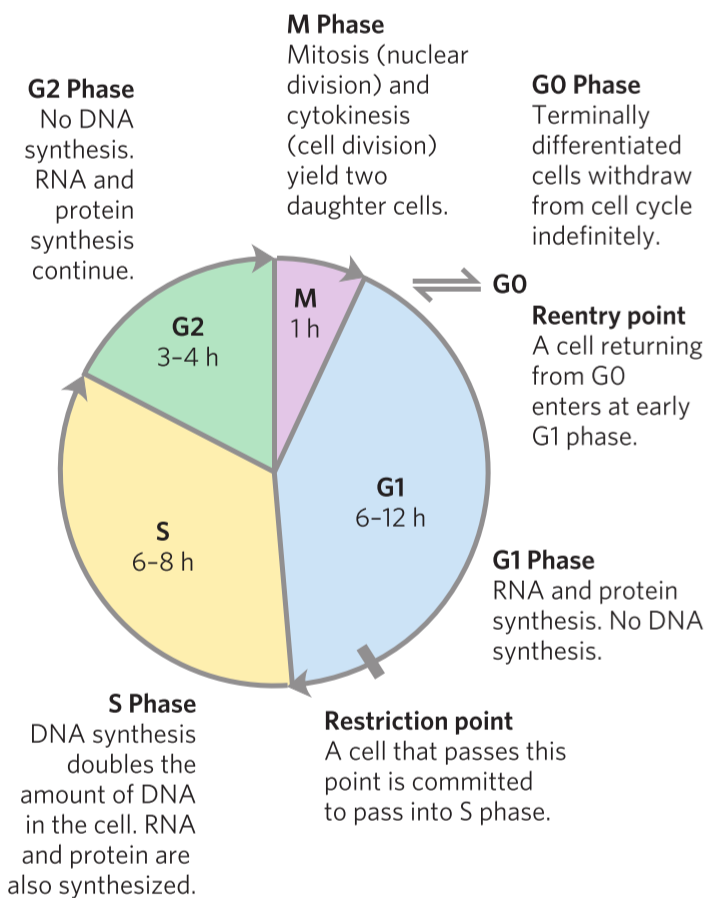
Many different cell types divide constantly. However, it is very important for cells to know when to divide and when not to. If a cell divides while it is in an unhealthy condition, cancer can occur. Proper cell division requires that the cell performs many “checkpoints” during the replication cycle, to make sure everything is working correctly before proceeding.
This replication cycle is called the cell cycle and has four well-defined stages. In the S phase, DNA is replicated. In the G2 phase, new proteins are synthesized. In the M phase (M for mitosis), the nuclear envelope breaks and the paired chromosomes are separated to two ends of the cell, and the cell is divided into two, producing two daughter cells. The cell now passes into G1 phase, where the cell can either enter the S stage to repeat the cycle or go into the G0 phase, where the cell stops dividing. Cells that don’t really divide during the lifetime, like cardiomyocytes and neurons can stay in the G0 for their lifetimes.
Regulating the cell cycle
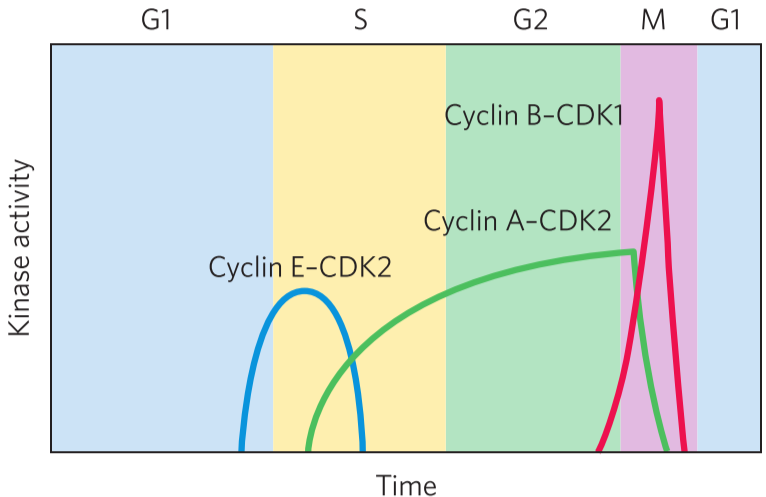
The cell cycle is regulated by oscillating values of specific protein kinases. These kinases phosphorylate proteins involved in the metabolic activities of the cell to control them. This ensures that the cell division proceeds correctly. These kinases are heterodimers that consist of two subunits: one cyclin subunit, that regulates the activity, and one CDK subunit that has the kinase activity. The CDK doesn’t work without binding a cyclin.
There are at least 10 different cyclin molecules (cyclin-A, cyclin-B …), and at least 8 different CDKs (CDK1, CDK2 …) They combine in many combinations at specific points in the cell cycle. The cell produces specific combinations of cyclin and CDK during specific phases to control the processes that should take place in each phase. For example, during the M phase, cyclin B and CDK 1 is produced by the cell, while the other cyclins and CDKs are inhibited or degraded. The cyclin-B-CDK1 heterodimer then phosphorylates and activates proteins that are needed for mitosis.
For this process to work, the correct cyclins and CDKs must be present, and the other cyclins and CDKs must be removed or inhibited. The cell uses four mechanisms to regulate the cyclin and CDKs. They are phosphorylation/dephosphorylation of CDK, degradation of CDK, periodic synthesis of CDKs and cyclins, and the action of specific CDK-inhibiting proteins.
Regulation of CDKs by phosphorylation
The activity of CDKs can be tightly controlled by phosphorylation and dephosphorylation of two specific residues on the protein. Phosphorylation of the 15th amino acid residue, a tyrosine (written as Tyr15) deactivates CDK2, while phosphorylation of the Thr160 residues activates the CDK. A phosphatase called PTPase dephosphorylates Tyr15, activating CDK.
The presence of single-strand breaks in DNA in a cell leads to the cell cycle stopping in G2 phase. A specific (unnamed) protein is activated by single-strand breaks and leads to the inactivation of PTPase, which means that CDK will remain inactivates. The cell can now repair the damage, and when the damage is repaired, the PTPase will be activated and dephosphorylate the Tyr15, so the CDK is activated and the cell cycle can proceed.
Controlled degradation of cyclin
The process of mitosis requires first the activation and then the destruction of cyclins A and B, which activate the M-phase CDK. These two cyclins contain an amino acids sequence called the “destruction box”. A protein called DBRP recognizes these destruction boxes and activates ubiquitin ligase, which attaches multiple ubiquitins to the cyclins, which causes them to be degraded by the proteasome.
During mitosis, the cyclin A and B and the M-phase CDK are activated. The CDK will then phosphorylate DBRP, which triggers the degradation of the cyclins that activate the CDK itself. This is a form of feedback inhibition.
Regulated synthesis of CDKs and cyclins
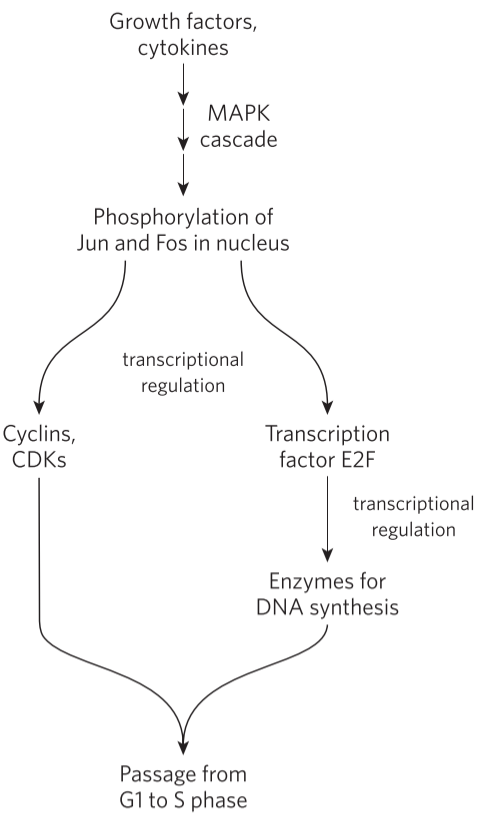
By regulating the synthesis of CDKs and cyclins, we can regulate their levels in the cell (obviously). For example, cyclin D, cyclin E, CDK2 and CDK4 are synthesized only in the presence of transcription factor E2F. This transcription factor is activated when growth factors and certain cytokines bind to the cell. The growth factor receptors phosphorylate two proteins called Jun and Fos, which act as transcription factors that induce transcription of E2F. The cyclins and CDKs activated by E2F induce the synthesis of specific nuclear transcription factors that are needed for producing the enzyme needed for DNA synthesis. This allows the cell to enter the S phase.
Inhibition of CDKs
CDKs are inhibited by many different proteins, like p16, p21, p27, p53 and p57. These proteins, and other CDK inhibiting proteins, are also tumor suppressor proteins; they prevent the cell from duplicating if something is wrong and by doing this, prevent tumors from forming. When the tumor suppressor proteins are absent or defective tumors will be formed. Two of the CDK inhibitors are detailed below. Tumor suppressors are explained in more detail in the next topic.
What do CDKs phosphorylate?
CDKs phosphorylate proteins that are needed for cell division, but what proteins specifically are phosphorylated? We don’t really know all of them yet, but we know some. The structure of the nuclear envelope is maintained partly by a protein called lamin. Lamin is phosphorylated by CDK, which causes it to disintegrate which causes the nuclear envelope to break. Actin and myosin, important proteins in dividing the duplicated cell into two daughter cells, are both inactivated by CDK.
Another very important substrate of CDKs is retinoblastoma protein, or pRb. When pRb is not phosphorylated, it binds the transcription factor E2F and inhibits it. E2F is then unable to promote transcription of proteins necessary for DNA replication, which makes the cell unable to pass from the G1 phase to the S phase. The cyclin E-CDK2 heterodimer can phosphorylate pRb which occurs when the cell receives a signal to proceed with the cell division.
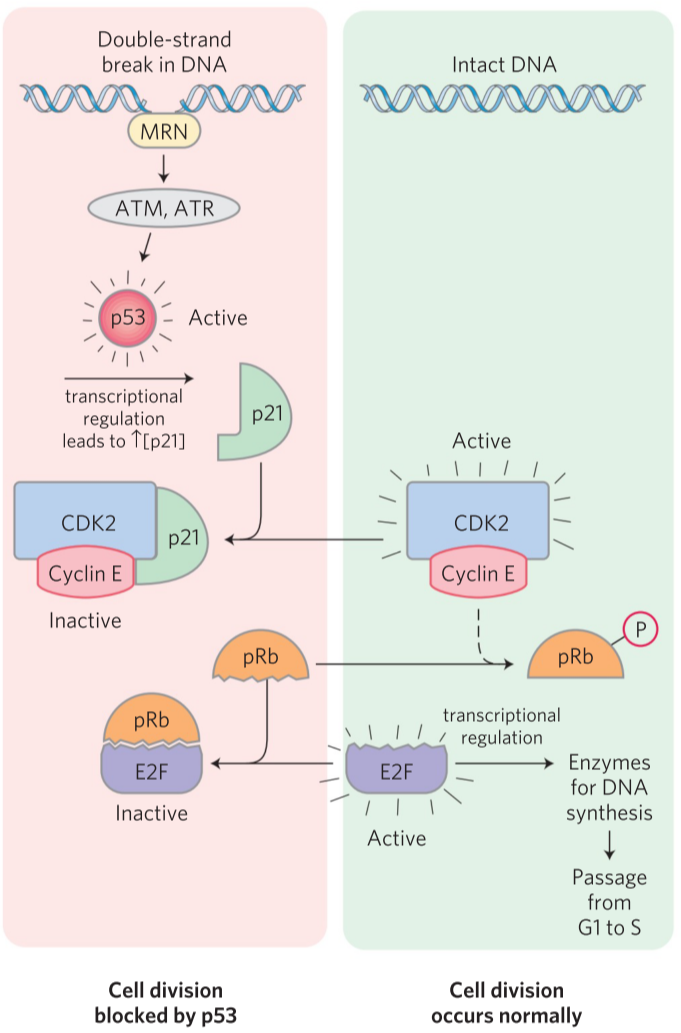
When there has been double-stranded DNA damage, the cell should not divide. A protein called MRN will bind to the double-stranded DNA damage and activate two kinases called ATM and ATR. They will phosphorylate and activate a protein called p53. p53 is a transcription factor that increases transcription of another protein, p21. This protein binds to the cyclin E-CDK2 heterodimer and inhibits it. Now that CDK2 is inhibited, pRb is never phosphorylated and will therefore bind and inactivate E2F. Now that E2F is inactivates, DNA synthesis enzymes will not be transcribed, and the cell is locked in G1 phase until the double-stranded DNA damage is fixed.