Page created on October 7, 2019. Last updated on December 18, 2024 at 16:55
Haemoglobin
General:
The red colour of RBCs comes from the fact that they contain a lot of haemoglobin (Hb). In fact, haemoglobin accounts for 95% of all proteins inside a red blood cell.
The function of haemoglobin is to carry oxygen in the blood. 1 gram of Hb can bind 1.39 mL of O2 gas. 99,7% of oxygen in the blood is transported via haemoglobin; only 0,3% of oxygen is dissolved in the blood.
There is a lot of haemoglobin in each RBC, so that 35% of the RBCs size comes just from haemoglobin. This is important, because if the body has problems synthesizing enough haemoglobin the RBCs will be smaller than normal (less than 7 µm).
The normal value of haemoglobin in the blood is 140 – 160 g/L. There is no free haemoglobin floating around in the plasma – all haemoglobin in the blood is inside RBCs. Normal RBC count in the blood is 5.0 – 5.5 million/µL in males and 4.5 – 5.0 million/µL in women.
Structure:
Haemoglobin is a protein that contains iron. Each molecule of haemoglobin can bind up to 4 molecules of oxygen (O2). It is comprised of 4 subunits and each subunit is comprised of a heme group and a globin polypeptide chain. The molecular weight is approximately 65 kDa. The heme group is the same in all stages of the life, but the structure of the globin units is different in adults and foetuses.
In adults 98% of haemoglobin is comprised of two α globin chains and two β globin chains. We usually write this as α2β2. The remaining 2% of haemoglobin is comprised of two α chains and two δ chains (α2δ2).
Because the foetal circulation is very different than the adult circulation foetuses have different haemoglobin than adults. Foetal haemoglobin is comprised of two α chains and two γ chains (α2γ2).
It is the heme group which contains the iron (Fe2+) ion. Each heme group can bind one molecule of O2, so one molecule of haemoglobin can bind 4 molecules of oxygen.
pH regulation:
Haemoglobin is a highly efficient pH buffer. The protein contains many histidine-residues which can take up or give away proteins, effectively buffering the blood.
Haemoglobin is a better pH buffer than plasma proteins because it exists in much higher concentration than other plasma proteins, and because it contains more histidine-residues than plasma proteins.
Gas compounds of haemoglobin:
Haemoglobin can bind other gases than O2, like CO2, CO and CN– (cyanide). When it binds to one of these gases its properties, name and spectroscopy (see physio lab) change.
When haemoglobin binds O2 it’s called oxygenated haemoglobin or oxy-haemoglobin. This is the normal haemoglobin found in high amounts in the arterial blood of a healthy person.
When haemoglobin binds CO2 it’s called deoxy-haemoglobin or reduced haemoglobin. It accounts for 1 – 5% of haemoglobin in a healthy person, but it accounts for 5 – 15% of haemoglobin in smokers. Unlike oxygenated haemoglobin (which is red) deoxygenated haemoglobin is blue. If the amount of deoxygenated haemoglobin increases above 50 g/L the skin and mucous membranes will become bluish-purplish discoloured. This is called cyanosis and can be seen in many diseases, but also on the lips of someone who is very cold.
When haemoglobin binds CO it’s called carboxy-haemoglobin or CO-haemoglobin. CO binds to haemoglobin 300 times easier than O2. When CO has bound to haemoglobin it doesn’t allow any O2 to bind to the same haemoglobin molecule. It also causes a left-shift of the haemoglobin-oxygen dissociation curve, which means that the haemoglobin can’t give away O2 to tissues that need it.
If the iron molecule in haemoglobin is oxidised from the normal Fe2+ form to the Fe3+ form the haemoglobin is called met-haemoglobin. This can occur in inherited diseases but also in nitrate poisoning.
If haemoglobin binds to cyanide ion (CN–) it’s called cyanmet-haemoglobin.
Disorders of haemoglobin synthesis:
Also called haemoglobinopathies, these are diseases where the structure of haemoglobin is abnormal. Many types exist.
Thalassaemia is a disorder where there is reduced synthesis of the globin chains. In α-thalassaemia there is reduced synthesis of the α-globin chains while in β-thalassaemia there is reduced synthesis of the β-globin chains.
Sickle cell disease is a condition where the structure of the β-chain is abnormal, which causes the RBCs to change shape from the normal biconcave shape to the shape of a sickle.
Metabolism of iron
The human body contains 4 – 5 grams of iron. It is distributed like this:
- 70% – in haemoglobin
- ~20% – in iron storages
- ~5% – in myoglobin
- ~5% – in enzymes (like cytochrome, peroxidase, catalase)
- ~1% – in the serum
Iron is continuously lost from the body in small amounts. The iron loss is larger in women due to menstruation, but both genders lose some iron through the GI tract. The GI mucosal cells store high amounts of iron, and these cells are shed every day. For this reason, we need a daily intake of iron to keep the iron level steady.
Free iron is toxic to cells, so iron should always be bound to proteins to keep the amount of free iron inside cells low. This prevents iron from killing the cells.
Iron is stored inside cells in the ferric (Fe3+) form as part of a protein called ferritin. Ferritin is a metalloprotein which is comprised of a protein called apoferritin and iron. Apoferritin forms the outer layer of the protein while the iron forms an iron core. 1 apoferritin molecule binds 4000 – 5000 iron atoms! Ferritin is water-soluble.
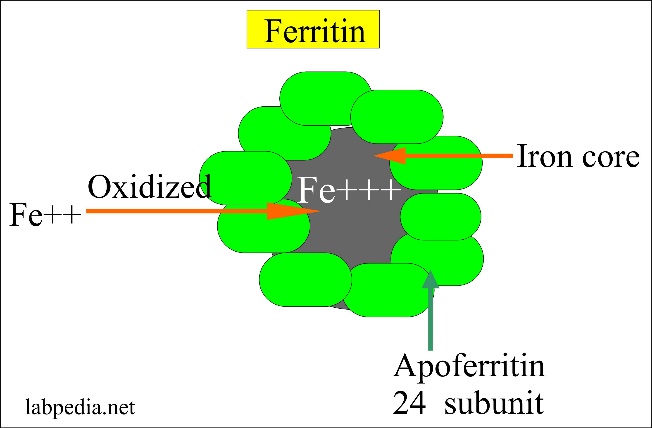
From http://www.labpedia.net/test/206
Iron is transported in the plasma as part of a protein called transferrin. Its composition is similar to that of ferritin. Transferrin is comprised of a protein called apotransferrin and iron in the ferric (Fe3+) form.
A small amount of iron in the body exists in a molecule called haemosiderin. This molecule is a breakdown product of ferritin, and it is not water soluble. The body cannot extract or utilize the iron inside haemosiderin, so this iron is “lost” to the body.
Iron absorption:
Iron is absorbed in the duodenum and proximal jejunum in the ferrous (Fe2+) form. Inside the GI mucosa cells iron binds to apoferritin to form ferritin.
Iron transportation:
When iron is to be transported into the blood it is removed from ferritin and attached to apotransferrin, forming transferrin. Transferrin circulates in the blood until it binds to a transferrin receptor, a protein that exists on the surface on cells. After this binding the cell will ingest the transferrin, and it can then take the iron from the transferrin molecule to use for itself.
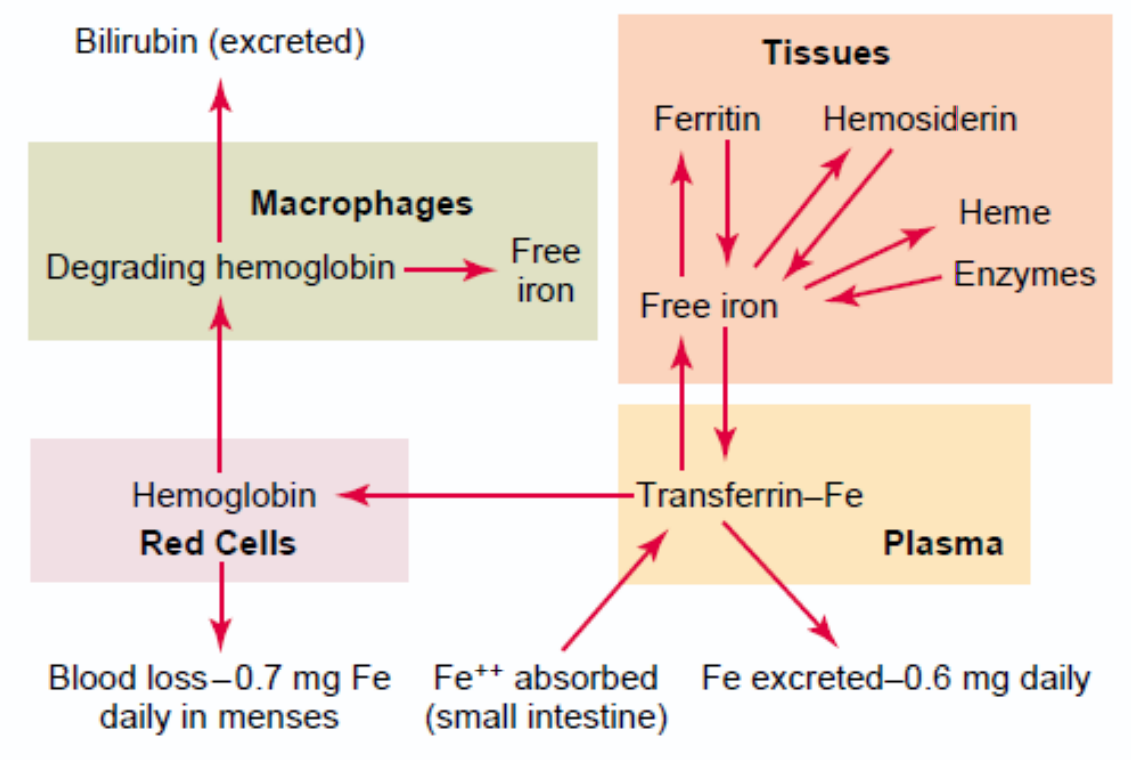
Credits to physiology department (?).
Degradation of erythrocytes and haemoglobin; bilirubin metabolism
RBCs are degraded in a part of the immune system called the reticuloendothelial system (RES) or the mononuclear phagocyte system (MPS). More specifically they’re destroyed by macrophages, mostly in the spleen.
The membrane part of the cell is degraded by macrophages in the spleen and liver. Haemoglobin is broken down into its components, the heme part and the globin part. The globin part is degraded into amino acids. The iron atom is release from the heme part and recycled. The porphyrin ring is metabolized into a molecule called indirect or unconjugated bilirubin. Bilirubin is a waste product the body should try to get rid of.
Unconjugated bilirubin is hydrophobic, so it can’t dissolve in blood and therefore can’t be transported in blood. To overcome this it binds to albumin, which allows it to be transported in the blood. Also, because it’s hydrophobic it can’t be excreted with the urine by the kidneys, so the liver must convert it into a hydrophilic form first.
When unconjugated bilirubin reaches the liver the hepatocytes (cells of the liver) will conjugate (attach) the unconjugated bilirubin with a molecule called glucuronic acid. The compound is now bilirubin-glucuronide. Because glucuronide is a hydrophilic group the whole bilirubin-glucuronide molecule is now hydrophilic. This form of bilirubin is called direct bilirubin or conjugated bilirubin.
Most of this bilirubin is excreted by the liver into the bile, while some is excreted by the kidneys into the urine. The bile is excreted into the duodenum, where it meets the gastrointestinal bacteria. These bacteria convert conjugated bilirubin into another compound called urobilinogen.
Approximately 50% of this urobilinogen is further converted into another compound called stercobilin. Stercobilin is brown and is the compound responsible for the colour of the faeces.
The other half of urobilinogen is absorbed by the gastrointestinal tract, causing it to return into the bloodstream. Here urobilinogen is converted into a compound called urobilin. Urobilin is yellow, and it is excreted by the kidneys into the urine. Urobilin is what gives urine the yellow colour.
Summary:
Haemoglobin is broken down into unconjugated bilirubin. Unconjugated bilirubin is conjugated in the liver to conjugated bilirubin. This is excreted in the bile and enters the GI tract. All unconjugated bilirubin is converted into urobilinogen in the GI tract. Half of the urobilinogen is converted into stercobilin and excreted with the faeces. The other half is absorbed and converted into urobilin in the blood. Urobilin is excreted by the urine.
Jaundice
Jaundice or icterus is the name of the pathological condition that occurs when the level of bilirubin (conjugated or unconjugated or both) in the serum is abnormally high. It causes the skin to become yellow.