Table of Contents
Page created on November 29, 2018. Last updated on November 15, 2021 at 21:48
Definition
Acute coronary syndrome (ACS) is an umbrella term for acute presentations of coronary artery disease or ischaemic heart disease. There are three types, each of which with differing underlying pathophysiology:
- Non-ST elevation coronary syndrome (NSTE-ACS)
- Unstable angina (UA)/unstable angina pectoris (UAP)
- Non-ST elevation myocardial infarction (NSTEMI)
- ST elevation myocardial infarction (STEMI)
Acute myocardial infarct (AMI) is a disorder which occurs when there is a lasting imbalance between perfusion and blood demand of myocardium. This imbalance is most commonly due to ischaemia, but can also occur in the context of normal perfusion but increased blood demand. AMI refers to the presence of either NSTEMI or STEMI.
AMI is a type of ischaemic heart disease, and it’s one of the acute coronary syndromes. It’s a clinical diagnosis, not a pathological one, and thus it’s defined by clinical evidence of acute myocardial injury together with clinical evidence of acute myocardial ischaemia.
Myocardial injury can be detected by elevated cardiac troponin values, while evidence of ischaemia can be detected by characteristic symptoms, characteristic ECG changes, or identification of a coronary thrombus.
Etiology
The majority of myocardial infarcts are are due to coronary artery disease, where atherosclerotic plaques form in the coronary arteries. When one such plaque ruptures the inside of the plaque is exposed to the blood. This is a thrombogenic surface, causing a thrombus to form on the plaque, totally or almost totally occluding the artery.
Some factors either increase the demand of the myocardium, or reduce the oxygen supply, which can aggravate the ischaemia:
- Hypertrophy
- Hypotension
- Shock
- Hypoxaemia
Causes other than coronary artery disease include:
- Embolus
- Vasculitis
- Coronary spasm
Pathogenesis
Ischaemia must last approx. 20 – 40 minutes to cause necrosis. This is a type of anaemic infarct. However, because AMI is a clinical diagnosis rather than a pathological one, and because of the fact that the symptoms and clinical signs of AMI appear later than the pathological changes, we can say that the acute myocardial infarction itself doesn’t occur until the ischaemia has lasted at least 2 – 4 hours. This means that by the time a person develops symptoms of AMI their myocardium has been ischaemic for 2 – 4 hours already.
Necrosis always begins in the subendocardial myocardium and extends towards the epicardium, potentially becoming transmural if the ischaemia is severe enough. This is because the arteries lie on the epicardium, so the subendocardial myocardium is the least perfused region.
Types
There are two types of myocardial infarction. If the coronary occlusion is complete the necrosis will be transmural, in which case it’s a type of transmural infarct. Transmural infarcts cause ST-segment elevated myocardial infarction (STEMI), where elevation of the ST-segments can be seen on ECG.
If the coronary occlusion is not complete but still severe enough to cause necrosis the necrosis will only be subendocardial. Subendocardial infarcts cause non-ST-segment elevated myocardial infarction (NSTEMI), where elevation of ST-segments is not present on the ECG. However, other ECG signs of ischaemia can be present.
Phases of myocardial infarct
The phases of myocardial infarct are best summed up in a table.
| Phase | Time-frame | Macroscopical characteristics | Microscopical characteristics | Potential complications |
| Hyperacute phase | 0 – 12 hours | Only visible with diaphorase reaction | Oedema, haemorrhage | Acute left ventricular failure if 80% of myocardium is affected. Lethal arrhythmias, like ventricular fibrillation. |
| Acute phase | 1 – 2 days | Yellowish, greyish. Clay-like | Signs of necrosis (no nuclei, eosinophilia) | |
| Recent phase | Day 2 – week 1 | Same as above, but with reddish demarcation zone | Neutrophil and macrophage infiltration. | Rupture of infarct, can cause pericardial tamponade. Rupture of papillary muscle causes acute mitral valve insufficiency. Fibrinous pericarditis. |
| Chronic (subacute) phase | Months, years | Scar tissue | Fibroblast involvement | Aneurysm of the scar tissue. Thrombosis inside aneurysm, potential embolization.
Chronic ischaemic heart disease. |
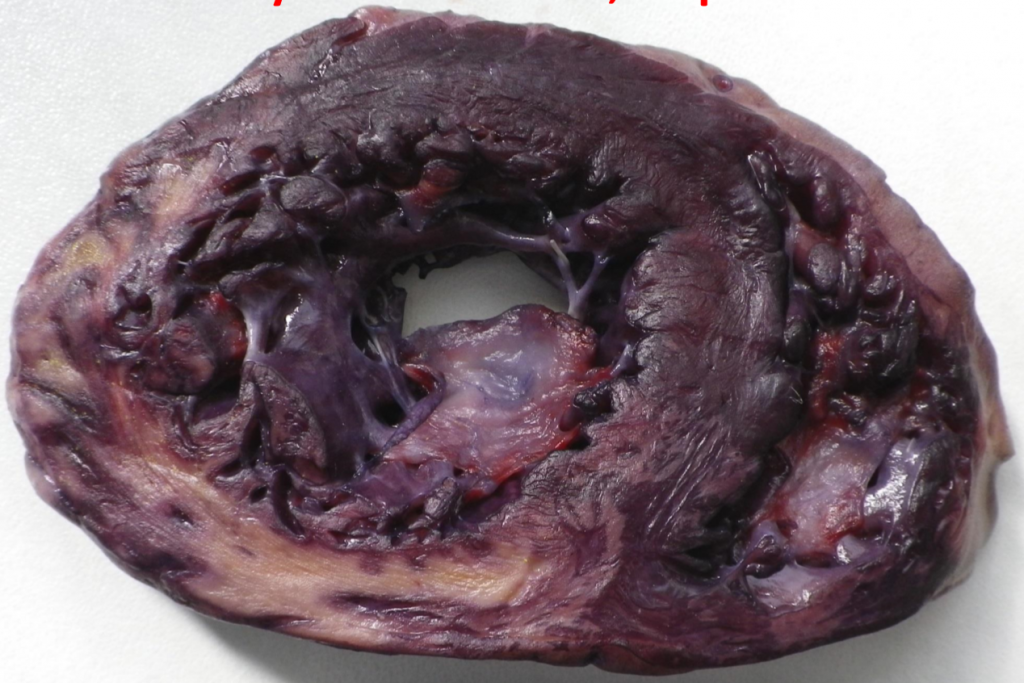
Complications
The complications of acute myocardial infarct can be divided into an early group and late group.
- Early (within 48 hours)
- Sudden cardiac death
- Arrhythmia
- Acute left ventricular failure
- Cardiogenic shock
- Late (after 48 hours and long-term)
- Rupture of the myocardial wall -> Pericardial tamponade
- Rupture of papillary muscles -> Mitral regurgitation
- Aneurysm of the myocardium
- Thrombosis and embolism
- Reinfarction
- Pericarditis
- Chronic ischaemic heart disease -> Heart failure
Diagnosis and treatment
As stated earlier, diagnosis is based on clinical evidence of myocardial injury and myocardial ischaemia. In practice, diagnosis is based on three factors:
- ECG changes
- ST elevation/depression
- Pathological Q waves
- Abnormal T-waves
- New-onset bundle branch blocks
- Characteristic chest pain
- A rise and/or fall in troponin levels in blood
Treatment
Treatment is based on trying to restore circulation as soon as possible; the earlier reperfusion the better. For this we can use:
- Inserting an inflatable balloon that dilates the occluded artery, done in percutaneous transluminal coronary angioplasty (PTCA, also called PCI)
- Taking the great saphenous vein or internal mammary artery and connecting it to the coronaries and aorta, called coronary bypass surgery.
- Thrombolytic drugs like streptokinase or tPA to break down the thrombus
Reperfusion
The goal of treatment in acute myocardial infarction is to salvage as much of the myocardium as possible, so tissue perfusion should be restored as early as possible. However, reperfusion can sometimes cause more bad than good when reperfusion injury occurs. This occurs by multiple factors:
Mitochondrial dysfunction occurs in the ischaemic myocardial cells. The mitochondrial membrane becomes more permeable, so osmotically activate components can enter the mitochondria and cause it to swell and burst. This releases mitochondrial contents that promote apoptosis.
During ischaemia will the intracellular calcium levels increase. After reperfusion myofibrils will contract uncontrollably, which can cause cytoskeletal damage and cell death.
During reperfusion will the ischaemic cells regain oxygen supply, but they’re so damaged that they use the oxygen to produce reactive oxygen species, which damages the cell membrane.
Leukocyte aggregation can occur during reperfusion, which can occlude the microvasculature and lead to the “no-flow” phenomenon. Platelet and complement activation also contribute to this phenomenon by damaging the endothelium.
Reperfusion arrhythmia is a potential complication of reperfusion, for unknown reasons. Reperfusion could induce dangerous arrhythmias like ventricular tachycardia and ventricular fibrillation.
Myocardial stunning is a phenomenon that may occur in myocardial cells that were ischaemic but reperfused before the ischaemia could irreversible damage the cell. In this postischaemic period the affected myocardium will be in a non-contractile state.
Hi Greek 🙂
Why is the affected myocardium in a non-contractile state in case of myocardial stunning. If there was no irreversible damage, isn’t the myocardium healthy?
I don’t really know what to say, to be honest. Even though an injury is reversible doesn’t mean it can’t induce temporary dysfunction.
There is a slight mistake on the first paragraph. Myocardial ischemia and infarct were confused with each other. It is myocardial ischemia itself, not AMI which occurs when there is imbalance between the blood supply and the demand of the myocardium, (and it’s also caused by reduced coronary blood flow, and everything else in that paragraph). The lecturer said that there is a difference between the two, and a myocardial ischemia doesn’t necessarily mean that there is a necrosis(myocardial infarct). Necrosis begins only after 20-40 min of myocardial ischemia and AMI occurs at least 2-4 hours of ischemia.
Reference: latest necrosis lecture video and slides (2020)
I’ve rephrased the topic slightly to be more accurate, with the unfortunate consequence of making it more confusing.
Looking forward to more comments!
Hyperacute phase should be the first hour of the ischemia
Do you have a source? I would love to agree with you but I’m not sure that’s correct.
The lecture states that no macroscopical changes can be seen the first 12 hours, so I would say that the hyperacute phase lasts until then. However the lecture doesn’t clearly provide a table of the different phases, and neither does Robbins, so I’d love if you could provide a reliable source.
As written in our notes from seminar, our teacher told us hyper acute is less than 1 hour.
In the notes from my teacher he stated that in the hyperacute phase, there are no microscopical or macroscopical changes as the signs of necrosis occur after 4 hours. So in the hyperacute phase the only way to check whether it’s undergone a MI would be the Diophorase reaction.. but then the acute phase would be 24 to 48 hrs that’s when we see signs of necrosis..
Not really got a source other than what was said in class.
But then what’s between hour 4 and hour 24? In my opinion should the phases be continuous, so it doesn’t make sense if the hyperacute phase ends at hour 4 and the next phase starts at hour 24.
I’ll ask my teacher for clarification on Monday.