Table of Contents
Page created on October 4, 2018. Last updated on December 18, 2024 at 16:57
We already went through the basic mechanisms of drug action in topic 3. Here we will discuss their signal mechanisms. The topic is mostly repetition of biochemistry 1 and 2.
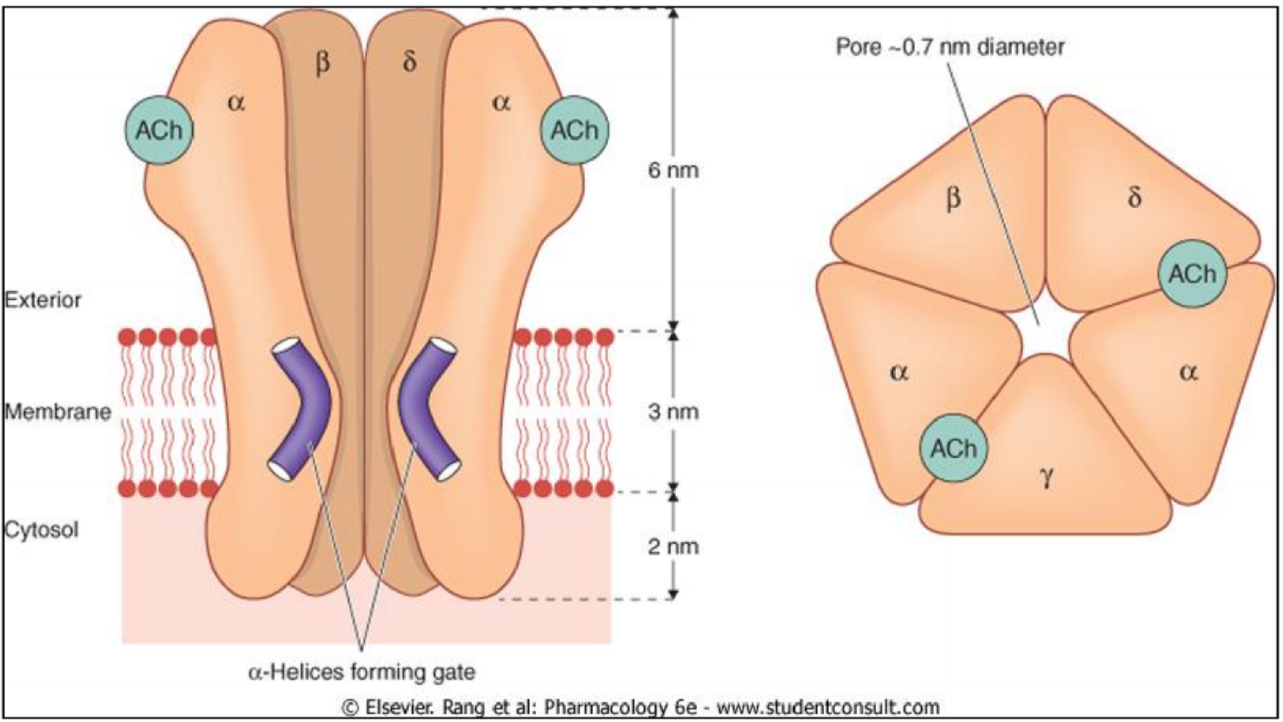
Ionotropic receptor
Ionotropic receptors, more commonly known as ligand-gated ion-channels, are ion channels that are opened when a ligand binds to it. This type of receptor consists of 4-5 subunits, usually comprised of α, β, γ, δ, and optionally other subunits too.
The signal transduction of ionotropic receptors is very fast, so drugs which act by ionotropic receptors act quickly. It usually happens in milliseconds. Once opened, ions will either enter the cell or leave it (depending on the ion), which causes either depolarization or hyperpolarization.
Examples of ionotropic receptors are the nicotinic acetylcholine receptor, the GABAA receptor or the 5-HT3 serotonin receptor.
Metabotropic receptors
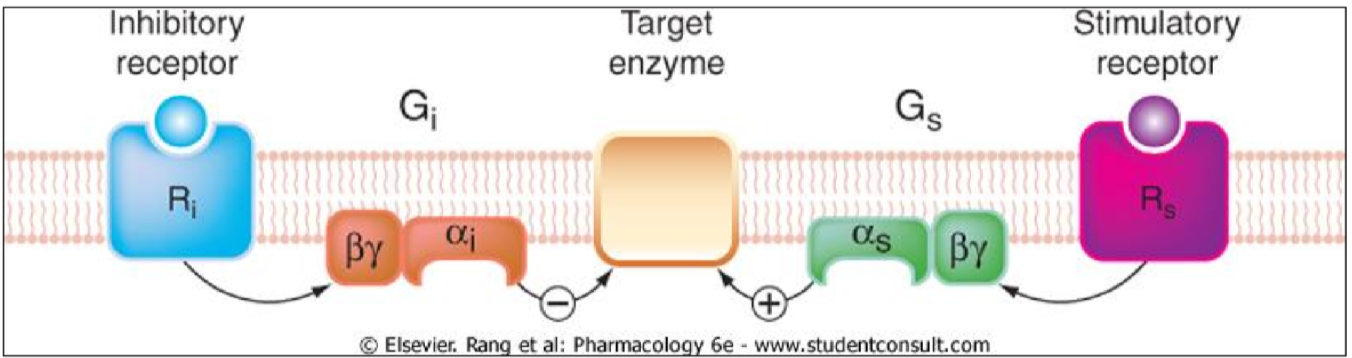
Metabotropic receptors, or G-protein coupled receptors, are membrane proteins that gets activated by a ligand-binding. Once activated, G-proteins on the inside of the cell get activated and can either stimulate or inhibit enzymes that produce second messengers. The G-proteins on the inside of the cell are built up of three subunits: The Gα-protein, the Gβ-protein and the Gγ-protein. The three are usually bound together in a trimer. The Gα-protein is normally bound to GDP.
When the receptor is activated, the Gα subunit will bind GTP instead of GDP, which activates it. The Gα subunit will then travel to a target enzyme and either activate it or inhibit it.
This means that, for example, when adrenaline binds to the β-receptor on a cell, adrenergic effects in the cell will increase. When adrenaline binds to the α2-receptor, adrenergic effects in the cell will decrease. These two receptors bind the same ligands (adrenaline, noradrenaline) but exert opposite changes in the cell.
The signal transduction of G-protein coupled receptors occurs in the range of seconds. This is slower than the ionotropic receptors and so drugs which act by metabotropic receptors may have slower action.
Three types of G-protein are important for us: Gi, Gs and Gq. The GS-protein activates adenylyl cyclase, Gi-protein inhibits adenylyl cyclase, and Gq-protein activate phospholipase C. Here is an overview of proteins using these G-proteins:
| GS-protein coupled receptors | Gi-protein coupled receptors | Gq-protein coupled receptors |
| Adenylyl cyclase activated | Adenylyl cyclase inhibited | Phospholipase C activated |
| Adrenergic β-receptor | Adrenergic α2-receptor | Adrenergic α1-receptor |
| Dopamine D1-receptor | Dopamine D2-receptor | Histamine H1-receptor |
| Histamine H2-receptor | Muscarinic type 2 (M2) receptors | Muscarinic type 1 and 3 (M1, M3) receptors |
Adenylyl cyclase
This enzyme converts ATP to cAMP, a second messenger. Adenylyl cyclase produces the second messenger which is responsible for these mechanisms in the body:
- Smooth muscle relaxation
- Cardiac muscle contraction
- Increase of neuronal excitability
- Increase of gastric acid secretion
As written above, the Gi-protein inhibits adenylyl cyclase (to the left on the figure), and the Gs-protein activates adenylyl cyclase (to the right on the figure).
After cAMP has been formed it will activate certain protein kinases like protein kinase A (PKA) or inhibit certain protein kinases like myosin light-chain kinase (MLCK).
In cardiac muscle cAMP will activate PKA which will open Ca2+-channels, allowing Ca2+ to flow into the cell. This Ca2+ causes the contraction.
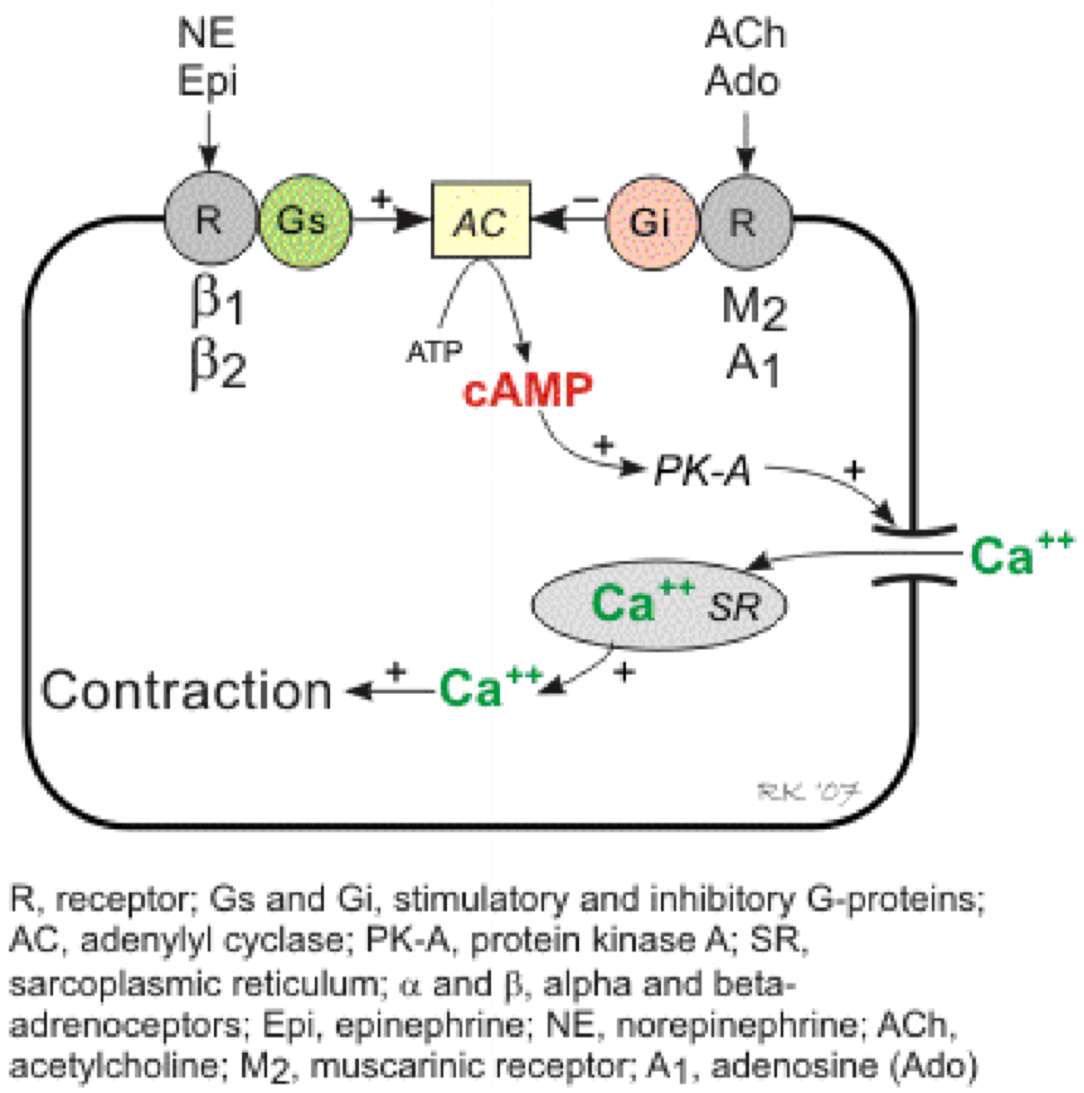
The signal transduction of cardiac muscle contraction. Note that norepinephrine and epinephrine bind to β1 and β2-receptors, which activates Gs which activates adenylyl cyclase, but acetylcholine and adenosine bind to muscarinic type 2 and adenosine type 1 receptors, which activates Gi protein which inhibits adenylyl cyclase.
Phospholipase C
This enzyme converts a molecule called PIP2 into DAG and IP3. DAG activates a protein kinase called protein kinase C (PKC), while IP3 opens Ca2+ channels on the ER or sarcoplasmic reticulum (SR), allowing Ca2+ to flow into the cytoplasm.
Phospholipase C produces the second messenger in these mechanisms in the body:
- Smooth muscle contraction
- Cardiac muscle contraction
- Endothelium-dependent vasodilation
- Neurotransmitter release
- Hormone secretion
We’ll look closer at smooth muscle contraction.
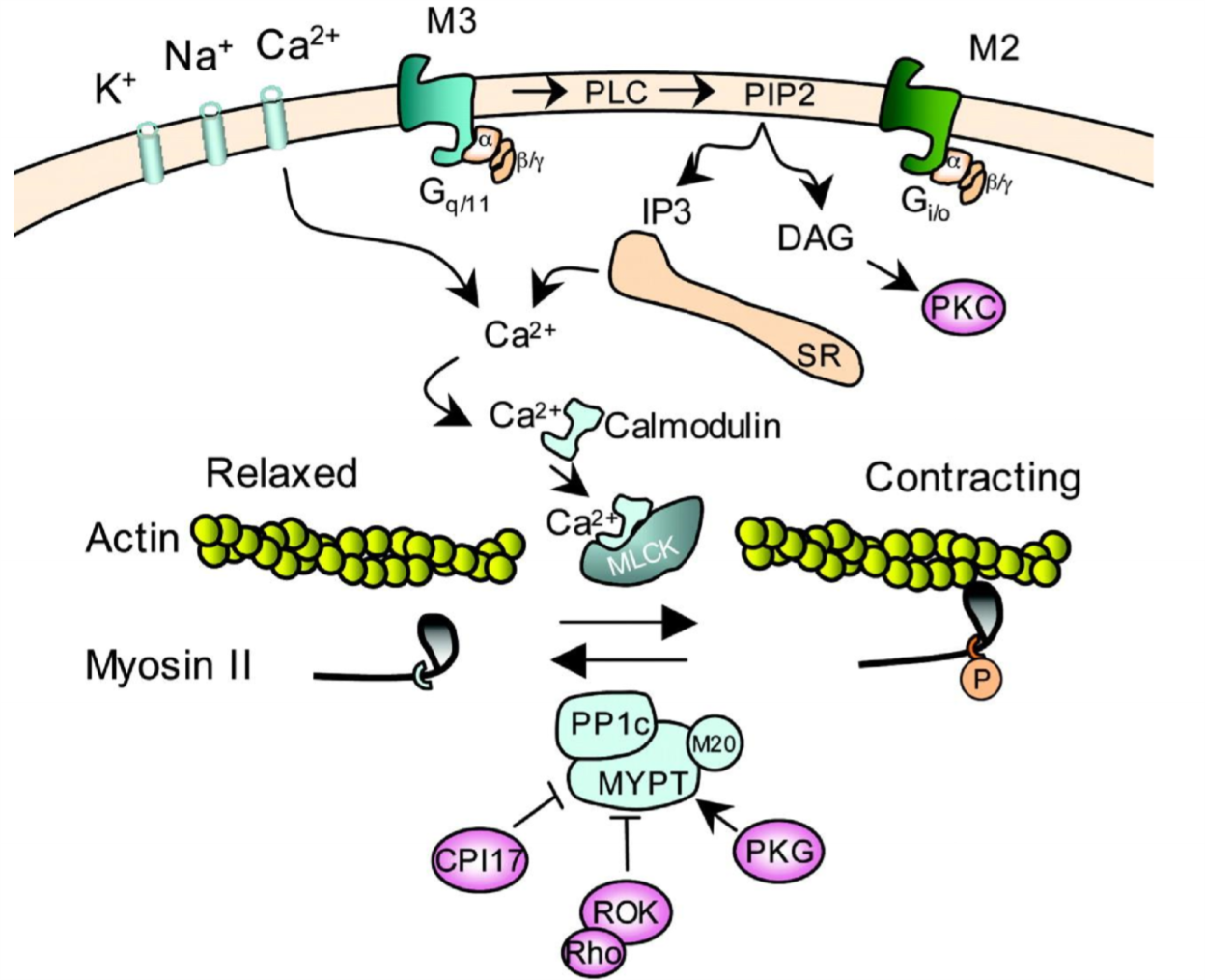
The first important thing here is that muscarinic type 3 (M3) receptors are activated. As you can see this receptor is coupled to a Gq-protein, meaning that activating the receptor will activate phospholipase C (PLC). This enzyme will cause formation of IP3, which will bind to an IP3-receptor on the sarcoplasmic reticulum (SR), which will cause Ca2+ to flow out of the SR into the cytoplasm. Ca2+ will bind to a protein called calmodulin, which will then bind to a protein called myosin light-chain kinase (MLCK), which will phosphorylate myosin and cause contraction.
Vasodilation is mostly controlled by the endothelium. When Ca2+ is released inside the cytoplasm of an endothelial cell by the same mechanism as in smooth muscle contraction, the Ca2+ will activate the enzyme eNOS, the endothelial NO synthase. This enzyme will produce NO, which will diffuse across the cell membrane into the smooth muscle cell in the tunica media of the vessel. Once inside the smooth muscle cell the NO-molecule will activate the enzyme guanylyl cyclase, which will increase the level of cGMP in the cell, which activates protein kinase G which eventually causes relaxation of the smooth muscle.
Phospholipase A2
Phospholipase A2 is an enzyme that converts phospholipids in the cell membrane into arachidonic acid. Arachidonic acid can be converted into various eicosanoids, either prostaglandins, leukotrienes or thromboxanes.
The eicosanoids can either work inside the cell as second messengers, or they can work as paracrine hormones by leaving the cell and affecting nearby cells.
Enzyme-linked receptors
These receptors are also enzymes. When they get activated, the receptors phosphorylate themselves, often on tyrosine residues. This phosphorylation is the beginning of the signal transduction.
The signal transduction of enzyme-linked receptors is slow; it takes minutes, up to 30 minutes even. Drugs which act by this mechanism can have slow action. Drugs which mimic endogenous compounds act on these receptors, like insulin, synthetic growth factors, synthetic cytokines, etc.
Enzyme-linked receptors can be either tyrosine kinase-linked (receptor tyrosine kinase), guanylate cyclase-linked, tyrosine-phosphatase-linked or serine/threonine kinase-linked.
The most famous enzyme-linked receptor is the insulin receptor, but growth factor and cytokines are also enzyme-linked.
Intracellular receptors
These receptors are not transmembrane proteins. Instead they reside inside the cytoplasm, so the ligand must be able to diffuse across the cell membrane in order to actually reach the receptor.
They have the slowest signal transduction; it takes many hours or even days! Drugs which act by this mechanism have the slowest onset of action. Some examples include:
- Retinoids
- Glucocorticoids
Examples of intracellular receptors are steroid hormone receptors, thyroid hormone receptors, vitamin D receptors and retinoid receptors.
Tachyphylaxis and tolerance
Tachyphylaxis:
Tachyphylaxis is an acute phenomenon that causes a drug to lose its effect on a patient in the short-term, like in minutes or hours. Tachyphylaxis can not be overcome by increasing the drug dose.
The following mechanisms can explain tachyphylaxis:
- Receptors are desensitized
- Receptor activation causes kinases to phosphorylate the of receptor. This phosphorylation activates a protein called Arrestin, which causes the receptor to be internalized.
- It’s a type of negative feedback
- The number of available receptors is reduced because other ligands occupy them
- An endogenous compound necessary for the drug action is depleted
Organic nitrates are notorious for causing tachyphylaxis.
Tolerance:
Tolerance is what occurs when someone takes morphine or cocaine or something over a long time and it eventually loses its effect, so the patient (or drug user) must increase the dose to achieve the same effect. Tolerance is a chronic phenomenon that causes a drug to lose its effect in the long-term, like in days or weeks.
The following mechanisms can explain how tolerance occurs:
- The number of receptors is downregulated
- Enzymes that metabolize the drug are stimulated
- The body counteracts the drug effects by using other mechanisms
- Like activating RAAS to preserve fluid when diuretics are given to a patient
- Drugs are more actively “pumped out” of the cells
- This occurs mostly against chemotherapeutical agents (as they are toxic to the cell)
- The body starts producing antibodies against the drug
Tolerance development is common for many drugs, like ethanol, opioids, psychoactive drugs, etc.
Hi Nik,
An example for a drug but also an endogenous compound acting on tyrosine-kinase linked receptors is insuline. 🙂
Greetz
Added a few examplez
Ok got it nm, thank u!
Hey!
cAMP causes smooth muscle CONTRACTION! I didn’t find anything supporting what you have written, in case it’s true please explain it further! Thanks
Under Ionotropic receptor it says:
“This type of receptor has 4-5 subunits: 2 α, 2 β and optionally 1 γ subunit.”
The picture of the receptor shows: 2 α, 1 β, 1δ and 1 γ.
what is correct?
Keep up the good work!
Good point. Different ionotropic receptors have different subunits. I changed the sentence now.