Table of Contents
Page created on March 5, 2018. Last updated on December 18, 2024 at 16:55
Learning objectives
- When is gluconeogenesis active?
- In which cellular compartments does gluconeogenesis take place?
- By which transporter does glucose leave the cell?
- What are the substrates of gluconeogenesis, and where do they originate from?
- In which organs does gluconeogenesis take place?
- What is the rate-limiting enzyme of the gluconeogenesis?
- Why do we need certain exclusive enzymes for the gluconeogenesis?
- Which enzymes of the gluconeogenesis are exclusive for it?
- Describe the Cori cycle
Introduction
Gluconeogenesis is the process where glucose is produced from various precursors. The glucose is then released into the bloodstream, which will travel to organs which need the glucose for energy.
Gluconeogenesis is only active during fasting, meaning between meals. Right after a meal the carbohydrates from the meal will be converted into glucose and released to the blood. after the carbohydrates from the meal have been consumed by cells, the body must supply glucose to the blood through gluconeogenesis (and glycogenolysis, next topic).
Gluconeogenesis not only supplies glucose for energy. Glucose and its derivatives are precursors of other important biomolecules like nucleotides and coenzymes. In fasting states gluconeogenesis provides glucose for these molecules as well.
Most enzymes of gluconeogenesis are in the cytosol, except for two enzymes: Pyruvate Carboxylase which is in the mitochondria and glucose 6-phosphatase which is in ER. PEP carboxykinase is found in both the cytosol and the mitochondria.
Many molecules can act as substrates for gluconeogenesis. Any substance which can be converted to oxaloacetate, like pyruvate, lactate, glycerol, and so-called glucogenic amino acids, can be used as substrates.
All amino acids are glucogenic, except leucine and lysine. These amino acids originate from breakdown of proteins; mainly from the food. After a few days of starvation, proteins of the body will start to be broken down for energy, too.
Glycerol can be acquired from the glycerol backbone of triacylglycerols, but the (even-numbered) fatty acids themselves can not be used as substrates. The only exception are odd-numbered fatty-acids, which can be converted to propionyl-CoA -> succinyl-CoA -> oxaloacetate. Acetyl-CoA can’t be converted to oxaloacetate and can therefore not be used as a substrate for gluconeogenesis.
Gluconeogenesis is mostly performed by the liver, but the renal cortex and the intestinal epithelium also perform it to some degree. Only these tissues have the enzyme glucose 6-phosphotase, so only they can perform gluconeogenesis.
Pathway
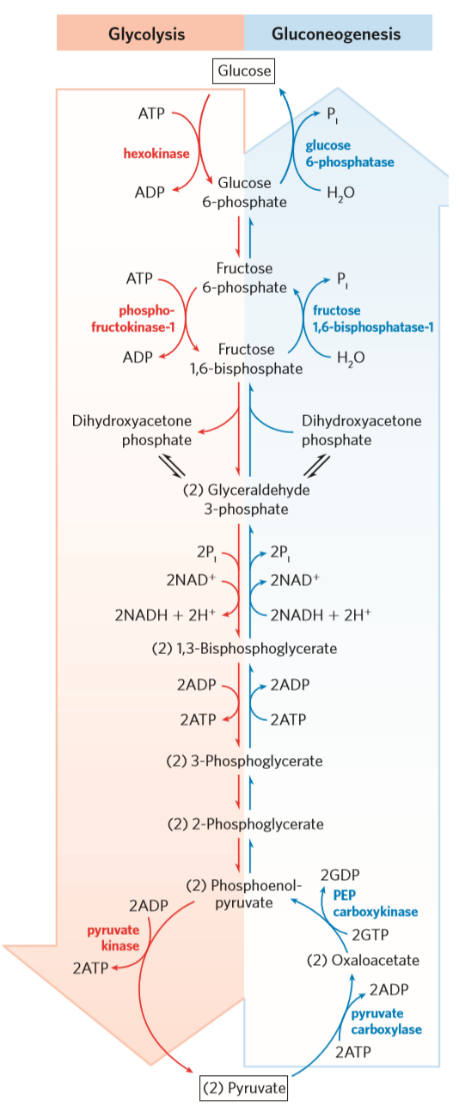
Pyruvate is transported from the cytosol into the mitochondria, where the pyruvate carboxylase converts pyruvate to oxaloacetate. However, oxaloacetate cannot be transported into the cytosol, as there is no transporter for it. OAA is therefore reduced to malate by mitochondrial malate dehydrogenase, passed into the cytosol as malate, and converted back into OAA in the cytosol by cytosolic malate dehydrogenase. Here, OAA is converted into phosphoenolpyruvate by PEP carboxykinase, which uses one GTP.
From PEP, gluconeogenesis follows the same path as glycolysis until it reaches fructose 1,6-bisphosphate. Here, fructose 1,6-bisphosphatase converts F16BP to fructose 6-phosphate. This is the rate-limiting enzyme of the gluconeogenesis.
Following the conversion of F6P to glucose 6-phosphate, glucose 6-phosphatase (found in ER) converts G6P to glucose. Glucose then leaves the cell through the GLUT2-transporter.
Lactate can be converted into pyruvate by lactate dehydrogenase. This pyruvate then follows the pathway outlined above.
The gluconeogenesis and glycolosis are just opposite pathways, so ideally they’d use the same enzymes, but in reverse. The gluconeogenesis does use 7 of the 10 enzymes used in glycolysis in reverse, but the remaining three enzymes cannot function in reverse in an energetically favourable manner (they’re irreversible). For this reason, gluconeogenesis uses 4 “bypassing” enzymes which are not found in the glycolysis.
The exclusive ones are Pyruvate carboxylase, PEP carboxykinase, Fructose 1,6-bisphosphatase-1 and Glucose 6-phosphatase. As an example, glucose 6-phosphatase and hexokinase catalyze the opposite reactions, but because hexokinase is irreversible glucose 6-phosphatase is needed.
Regulation
Running glycolysis and gluconeogenesis at the same time would be a complete waste of energy, as they catalyse opposite reactions. As both processes happen in the cytosol, they are reciprocally (oppositely) regulated, so that when one runs, the other stops. Regulation is covered in a later lecture.
Cori cycle
When skeletal muscle performs anaerobic glycolysis during exercise, lactate is produced. Lactate is then transported by the blood to the liver. The liver converts lactate into glucose by gluconeogenesis. The glucose will then travel back to the blood and then to the muscles, to provide energy again. This cycle is called the Cori cycle, and it is one of the most important providers of substrate for the gluconeogenesis.
The Cori cycle also occurs between RBCs and the liver. As RBC’s don’t have mitochondria, they can only produce energy anaerobically (without oxidative phosphorylation) from glucose via anaerobic glycolysis, which yields lactate.
A similar cycle called the alanine cycle transports both ammonia and pyruvate to the liver from the muscle. This cycle is detailed in topic 11.
Fates of pyruvate
Pyruvate has many possible fates in the liver:
- Conversion to oxaloacetate to be used in gluconeogenesis
- By pyruvate carboxylase
- Conversion to alanine
- By alanine transaminase
- Conversion to lactate
- By lactate dehydrogenase
- Conversion to acetyl-CoA to be used as a substrate for the TCA cycle
- By pyruvate dehydrogenase complex
Conversion to acetaldehyde by pyruvate decarboxylase occurs in yeasts but not in humans.
Summary
- When is gluconeogenesis active?
- When fasting, between meals
- In which cellular compartments does gluconeogenesis take place?
- Mostly in the cytosol
- Except Pyruvate Carboxylase which is in the mitochondria and G6Pase which is in ER
- By which transporter does glucose leave the cell?
- GLUT2
- What are the substrates of gluconeogenesis, and where do they originate from?
- Glucogenic amino acids – from breakdown of muscle
- Pyruvate
- Lactate – from Cori cycle
- Glycerol – from triacylglycerols
- Proprionyl-CoA – from odd-chained fatty acids
- In which organs does gluconeogenesis take place?
- Mainly liver, but also adrenal cortex and intestinal epithelium
- What is the rate-limiting enzyme of the gluconeogenesis?
- Fructose 1,6-bisphosphatase
- Why do we need certain exclusive enzymes for the gluconeogenesis?
- Because 3 enzymes of the glycolysis are irreversible, so we need “bypassing” reaction
- Which enzymes of the gluconeogenesis are exclusive for it?
- Pyruvate carboxylase, PEP carboxykinase, Fructose 1,6-bisphosphatase-1 and Glucose 6-phosphatase
- Describe the Cori cycle
- The Cori cycle transports lactate from muscles to the liver, where gluconeogenesis converts it back to glucose
- Which are the possible fates of pyruvate in the liver?
- Conversion to oxaloacetate, alanine, lactate, acetyl-CoA
I am wondering about something: Is the acetaldehyde-reaction happening in the liver? I thought it was only occuring in yeast.. 😅
Correct! Fixed now.